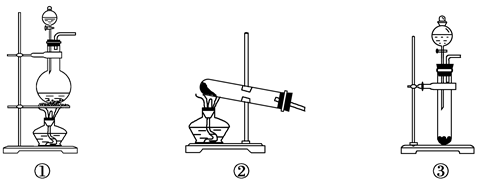
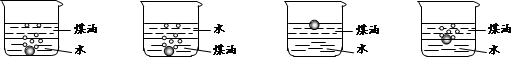��Ŀ����
��ѧ��ȤС���ͬѧΪ�ⶨijNa2CO3��NaCl�Ĺ���������Ʒ��Na2CO3��������������������ʵ�飬������벢��ɶ��й�����Ľ��
ͼ1 ͼ2
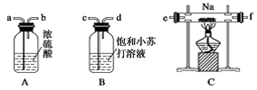
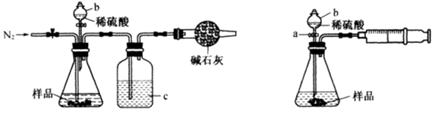
��1����ͬѧ��ͼ1��ʾװ�òⶨCO2��������ʵ��ʱϡ����������Ʒ�е� (�Na2CO3����"NaCl��)������Ӧ������b�������� ��ϴ��ƿc��ʢװ����Ũ���ᣬ��Ũ����������� ��
��2����ͬѧ��ͼ2��ʾװ�ã�ȡһ����������Ʒ(Ϊm g���Ѳ��)������ϡ���ᷴӦ����ʵ�飬�����Ʒ��Na2CO3�����������IJⶨ��
��ʵ��ǰ������װ�������Եķ������ȴ���a����bע��ˮ�����¶˲��������γ�һ��ˮ�����ٽ���Ͳ����������ѹ����b�¶˲������е� ��������װ�����������á�
����ʵ�����ʱ����ֱ�Ӳ�õ�������CO2�� (������������������)��
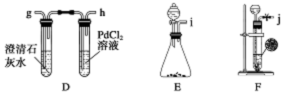
��3����ͬѧ����ͼ��ʾ�����Ͳ���ʵ�飺
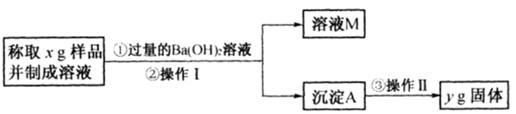
�ٲ���I�漰��ʵ�������� ��ϴ�ӣ��������漰��ʵ�������и�� ��
�ڱ���õ���Ʒ��Na2CO3���������ļ���ʽΪ ��
��4����״���£���672 mL CO2��ͨ��50 mL1mol/LKOH��Һ�У���ȫ��Ӧ��������Һ��K2CO3��KHCO3�����ʵ���֮��Ϊ(�跴Ӧǰ����Һ����仯���Բ���) ��

ͼ1 ͼ2
��1����ͬѧ��ͼ1��ʾװ�òⶨCO2��������ʵ��ʱϡ����������Ʒ�е� (�Na2CO3����"NaCl��)������Ӧ������b�������� ��ϴ��ƿc��ʢװ����Ũ���ᣬ��Ũ����������� ��
��2����ͬѧ��ͼ2��ʾװ�ã�ȡһ����������Ʒ(Ϊm g���Ѳ��)������ϡ���ᷴӦ����ʵ�飬�����Ʒ��Na2CO3�����������IJⶨ��
��ʵ��ǰ������װ�������Եķ������ȴ���a����bע��ˮ�����¶˲��������γ�һ��ˮ�����ٽ���Ͳ����������ѹ����b�¶˲������е� ��������װ�����������á�
����ʵ�����ʱ����ֱ�Ӳ�õ�������CO2�� (������������������)��
��3����ͬѧ����ͼ��ʾ�����Ͳ���ʵ�飺

�ٲ���I�漰��ʵ�������� ��ϴ�ӣ��������漰��ʵ�������и�� ��
�ڱ���õ���Ʒ��Na2CO3���������ļ���ʽΪ ��
��4����״���£���672 mL CO2��ͨ��50 mL1mol/LKOH��Һ�У���ȫ��Ӧ��������Һ��K2CO3��KHCO3�����ʵ���֮��Ϊ(�跴Ӧǰ����Һ����仯���Բ���) ��
��1��Na2CO3 (1��) ��Һ©��(1��) ��ȥCO2�е�ˮ����(1��)
��2����Һ��(1��)�����(1��)
��3���ٹ���(1��) ����(1��) ��106y/197x (1��)
��4��n(K2CO3):n(KHCO3)=2:1(2��)
��2����Һ��(1��)�����(1��)
��3���ٹ���(1��) ����(1��) ��106y/197x (1��)
��4��n(K2CO3):n(KHCO3)=2:1(2��)
�����������1�� NaCl����ϡ���ᷴӦ����ѡNa2CO3������b�������Ƿ�Һ©����Ũ����������dz�ȥCO2�е�ˮ�����������CO2���壩����2�� �ٽ���Ͳ����������ѹ�������������е�ѹǿ������b�¶˲������е�Һ����������װ�����������á���CO2�����壬����ֱ�Ӳ�õ�������CO2���������3�� �ٳ������ɣ��ʲ���I��Ҫ�漰���˲�����Ҫ֪�������������Ҫ���ء��ھ�����ѧʽ�ļ��㣬��Ʒ��Na2CO3���������ļ���ʽΪ106y/197x����4�������ķ�ӦΪ0.03CO2+0.05KOH=XK2CO3+YKHCO3+0.025H2O��X+Y=0.03��2X+Y=0.05�����X=0.02��Y=0.01������Һ��K2CO3��KHCO3�����ʵ���֮��Ϊn(K2CO3):n(KHCO3)0.02��0.01=2:1��2CO3����Ʒ��Na2CO3�������������漰����ԭ������ѧ̽�����̣���һ���ۺ���ǿ�ĺ��⡣

��ϰ��ϵ�д�
�����Ŀ