��Ŀ����
����Ŀ�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
��1����ͼ��![]() ��
��![]() ��Ӧ����
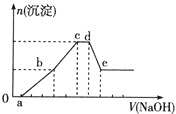
��Ӧ����![]() �����������ı仯ʾ��ͼ�������й�˵����ȷ����_______��
�����������ı仯ʾ��ͼ�������й�˵����ȷ����_______��
a. ��Ӧ��������������������������
b. ��Ӧ��Ļ�ܱ�������Ļ�ܸ�
c. ��Ӧ����ܼ��ܱ���������ܼ��ܸ�
d. �÷�ӦΪ������Ӧ
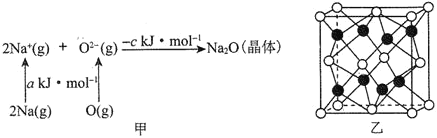
��2�����![]() ��
��![]() ��Ӧ���Ȼ�ѧ����ʽ��_______�������÷�Ӧ���з������Ҫ�о�Ϊ________��
��Ӧ���Ȼ�ѧ����ʽ��_______�������÷�Ӧ���з������Ҫ�о�Ϊ________��
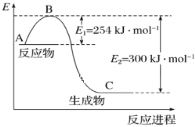
��3���Ը��ݱ��м�ͼ�����ݼ���![]() �ļ���______________ kJ/mol��
�ļ���______________ kJ/mol��
��ѧ�� |
|
|
����/ kJ/mol | 390 | 943 |
��4����![]() ����ԭ
����ԭ![]() ���������������������Ⱦ����֪��
���������������������Ⱦ����֪��
4NH3(g)+3O2(g)=2N2(g)+6H2O(g)����H1=-akJ/mol
N2(g)+O2(g)=2NO(g)����H2=-bkJ/mol
��1molNH3��ԭNO��N2����÷�Ӧ�����еķ�Ӧ����H3=_____________kJ/mol(�ú�a��b��ʽ�ӱ�ʾ)��
���𰸡�a ![]() ���������ɣ� ���оݻ���H<0 435
���������ɣ� ���оݻ���H<0 435 ![]()
��������
(1)����ͼ�÷�Ӧ�������������������������ߣ����N2(g)+3H2(g)2NH3(g)�����жϣ�
(2)������˷�Ӧ���ʱ䣬�����Ȼ�ѧ����ʽ����д������д���Ȼ�ѧ����ʽ���÷�Ӧ����������ʵ������٣���ϸ����оݷ������
(3)���ݷ�Ӧ�ȵ��ڷ�Ӧ����ܼ��ܼ�ȥ��������ܼ��ܼ��㣻
(4)���ø�˹���ɷ������㡣
(1)����ͼ��Ӧ�������������������������ߣ�˵���÷�ӦΪ���ȷ�Ӧ����Ӧ����ܼ���С����������ܼ��ܣ���Ӧ��Ļ��Ϊ254 kJ/mol��������Ļ��Ϊ300 kJ/mol����Ӧ��Ļ�ܱ�������Ļ�ܵͣ���Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2(g)2NH3(g)������Ӧ��һ���ؼ�С�ķ�Ӧ����ȷ��ֻ��a���ʴ�Ϊ��a��
(2)��Ӧ��������������������������ӦΪ���ȷ�Ӧ������1mol�����ų�46kJ��������Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2(g)2NH3(g)��H=-92kJ/mol���÷�Ӧ��һ���ؼ�С�ķ�Ӧ�������÷�Ӧ���з������Ҫ�о�Ϊ���оݣ��ʴ�Ϊ��N2(g)+3H2(g)2NH3(g)��H=-92kJ/mol�����оݣ�
(3)��Ӧ�ȵ��ڷ�Ӧ����ܼ��ܼ�ȥ��������ܼ��ܣ���H-H�ļ���Ϊx����943 kJ/mol +3 x-6��390 kJ/mol =-92 kJ/mol��x=435 kJ/mol���ʴ�Ϊ��435��
(4)��4NH3(g)+3O2(g)=2N2(g)+6H2O(g)����H1=-akJ/mol����N2(g)+O2(g)=2NO(g)����H2=-bkJ/mol�����ݸ�˹���ɣ���![]() �ɵã�NH3(g)+
�ɵã�NH3(g)+![]() NO(g)=
NO(g)=![]() N2(g)+
N2(g)+![]() H2O(g)��H3=
H2O(g)��H3=![]() kJ/mol���ʴ�Ϊ��
kJ/mol���ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�