��Ŀ����
����Ŀ��B��N��Ti��Fe������Ҫ�IJ���Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�á�
��1����̬Fe2���ĵ����Ų�ʽΪ_____��Tiԭ�Ӻ����________���˶�״̬��ͬ�ĵ��ӡ�
��2��BH3������NH3���ӵĿռ�ṹ�ֱ�Ϊ_________��BH3��NH3��Ӧ���ɵ�BH3��NH3�����к��еĻ�ѧ��������_______����BH3��NH3��Bԭ�ӵ��ӻ���ʽΪ________��
��3��N��Pͬ���塣��ѧ��Ŀǰ�ϳ���N4���ӣ��÷�����N��N��N���ļ���Ϊ________��N4�ֽ���ܲ���N2���ͷų������������Ʋ�����;___________��(д��һ�ּ���)
��4��NH3��Cu2�����γ�[Cu(NH3)4]2�������ӡ���֪NF3��NH3������ͬ�Ŀռ乹�ͣ���NF3������Cu2���γ������ӣ���ԭ����____��
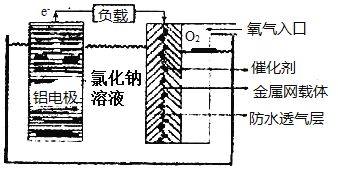
��5������TiO2��һ��Ӧ�ù㷺�Ĵ����������һ��ʵ����ͼ��ʾ���������ҵķе����Ը��ڻ�����ף���Ҫԭ����______�����������в�ȡsp3�ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________��
���𰸡�1s2 2s2 2p6 3s2 3p6 3d6 ��[Ar]3d6 22 ƽ���������Ρ������� ���ۼ�����λ�� sp3 60�� �������ƽ�����ըҩ(���������𰸾���) F �ĵ縺�Ա� N ��N��F �ɼ����Ӷ�ƫ�� F������ NF3 �е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ�� �������ҷ��Ӽ������� N>O>C
��������
(1)��Ϊ26��Ԫ�أ�ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+���ݴ���д��������Ų�ʽ�������ÿ�����ӵ��˶�״̬����ͬ��
(2)NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
(3)N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�ÿ�����Ϊ�������Σ�N4�ֽ���ܲ���N2���ͷų������������ݴ��ж���;��
(4)NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�
(5)����Ĵ��ڵ��������۷е����ߣ��������������γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ��ݴ�����
(1)��ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d6��[Ar]3d6��TiԪ��ԭ�Ӻ�����22�����ӣ�����ԭ�����˶�״̬��ͬ�ĵ��ӹ���22�֣��ʴ�Ϊ��1s22s22p63s23p63d6��[Ar]3d6��22��
(2)NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�BF3��Bԭ�Ӻ���3�������Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�NH3��Nԭ�Ӻ���3��������1���µ��Ӷԣ�����NH3Ϊ�������ͣ�BF3NH3�����к��еĻ�ѧ�������й��ۼ�����λ������BF3NH3��Bԭ�ӵļ۲���Ӷ���Ϊ4+0=4���ӻ���ʽΪsp3���ʴ�Ϊ��ƽ���������Σ������ͣ����ۼ�����λ����sp3��
(3)N4������Nԭ���γ�3������������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���ÿ����Ϊ�������Σ�N-N ���ļ���Ϊ60����N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ���ʴ�Ϊ��60�����������ƽ�����ըҩ��
(4)F�ĵ縺�Դ���NԪ�أ�NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�����NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ��Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�����NF3������Cu2+�γ������ӣ��ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��F������NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ����
(5)����Ĵ��ڻᵼ�������۷е����ߣ����к��з��Ӽ�������ײ�����������Ի��������۷е���ڼף��������������γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������N��O��C���ʴ�Ϊ���������ҷ��Ӽ���������N��O��C��