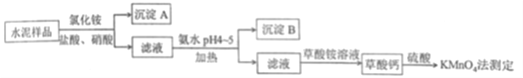
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΔώ.Υ°Ρύ «÷Ί“ΣΒΡΫ®÷ΰ≤ΡΝœΓΘΥ°Ρύ λΝœΒΡ÷ς“Σ≥…Ζ÷ΈΣCaOΓΔSiO2Θ§≤ΔΚ§”–“ΜΕ®ΝΩΒΡΧζΓΔ¬ΝΚΆΟΨΒ»Ϋπ τΒΡ―θΜ·ΈοΓΘ Β―ι “≤βΕ®Υ°Ρύ―υΤΖ÷–ΗΤΚ§ΝΩΒΡΡ≥÷÷ΖΫΖ®Ιΐ≥Χ»γΆΦΥυ ΨΘΚ
Θ®1Θ©‘ΎΖ÷ΫβΥ°Ρύ―υΤΖΙΐ≥Χ÷–Θ§“‘―ΈΥαΈΣ»ήΦΝΘ§¬»Μ·οßΈΣ÷ζ»ήΦΝΘ§ΜΙ–ηΦ”»κΦΗΒΈœθΥαΓΘΦ”»κœθΥαΒΡΡΩΒΡ «_____________Θ§ΜΙΩ… Ι”Ο__________¥ζΧφœθΥαΓΘ
Θ®2Θ©≥ΝΒμAΒΡ÷ς“Σ≥…Ζ÷ «________Θ§Τδ≤Μ»ή”Ύ«ΩΥαΒΪΩ…”κ“Μ÷÷»θΥαΖ¥”ΠΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________ΓΘ
Θ®3Θ©Φ”Α±Υ°Ιΐ≥Χ÷–Φ”»»ΒΡΡΩΒΡ «_____ΓΘ≥ΝΒμBΒΡ÷ς“Σ≥…Ζ÷ΈΣ_______ΓΔ_________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
ΔρΓΔ“―÷Σ25Γφ ±≤ίΥαΒΡΒγάκ≥Θ ΐΈΣK1=5.0ΓΝ10-2,K2=5.4ΓΝ10-5.≤ίΥαΗΤΒΡKap=4.0ΓΝ10-8.ΧΦΥαΗΤΒΡKsp=2.5ΓΝ10-9Θ°≤ΜΆ§Έ¬Ε»œ¬Υ°ΒΡάκΉ”Μΐ≥Θ ΐΦϊœ¬±μΘΚ
t/Γφ | 0 | 10 | 20 | 25 | 40 | 50 | 90 | 100 |
Kw/10-14 | 0.134 | 0.292 | 0.681 | 1.00 | 2.92 | 5.57 | 38.0 | 55.0 |
Θ®1Θ©ΦΤΥψ25Γφ ±KHC2O4»ή“ΚΒΡΥ°ΫβΤΫΚβ≥Θ ΐKb=_____ΘΜ–¥≥ωΥ°»ή“Κ÷–≤ίΥαΒΡΒγάκΖΫ≥Χ Ϋ_____ΘΜ≥ΘΈ¬œ¬ΫΪ0.2mol/LΒΡKOH»ή“Κ10mL”κ0.2mol/LΒΡ≤ίΥα»ή“Κ20mLΜλΚœΚσ»ή“Κœ‘________–‘ΓΘΘ®ΧνΓΑΥα–‘Γ±ΓΑ÷––‘Γ±ΜρΓΑΈόΖ®»ΖΕ®Γ±Θ©
Θ®2Θ©25Γφ ±œρ20mL≤ίΥαΗΤΒΡ±ΞΚΆ»ή“Κ÷–÷πΒΈΦ”»κ8.0ΓΝ10-4mol/LΒΡΧΦΥαΦΊ»ή“Κ10mLΘ§ΡήΖώ≤ζ…ζ≥ΝΒμΘ§_______Θ®ΧνΓΑΡήΓ±ΜρΓΑΖώΓ±Θ©Θ° ΧΦΥαΗΤΉΣΜ·ΈΣ≤ίΥαΗΤΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______Θ§Ν– ΫΦΤΥψΗΟΖ¥ΒΡΤΫΚβ≥Θ ΐK=_________ΓΘ
Θ®3Θ©90Γφ ±Θ§ΫΪ0.005mol/LΒΡ«β―θΜ·ΗΤ»ή“Κ20mL”κ0.0012mol/LΒΡ≤ίΥα»ή“Κ20mLΜλΚœΘ§‘ρΜλΚœΚσ»ή“ΚΒΡpH______ΓΘΘ®Ω…Ρή”ΟΒΫΒΡΕ‘ ΐΘΚlg38=1.6Θ§lg26=2.4Θ§lg2=0.3Θ©
ΓΨ¥πΑΗΓΩ ―θΜ·―«ΧζάκΉ” H2O2ΓΔ¬»Υ°Β» SiO2 SiO2 + 4HF === SiF4Γϋ+ 2H2O ¥ΌΫχFe3+ΓΔAl3+Υ°ΫβΘ§Φ”ΥΌ≥ΝΒμ…ζ≥… Fe(OH)3 Al(OH)3 2ΓΝ10-13 H2C2O4![]() H++HC2O4-Θ§HC2O4-
H++HC2O4-Θ§HC2O4-![]() H++C2O42- ΓΨ¥πΧβΩ’10ΓΩΥα–‘ Ζώ CaCO3(s) + C2O42- (aq)
H++C2O42- ΓΨ¥πΧβΩ’10ΓΩΥα–‘ Ζώ CaCO3(s) + C2O42- (aq) ![]() CaC2O4(s) + CO32- (aq)
CaC2O4(s) + CO32- (aq) ![]() 10
10
ΓΨΫβΈωΓΩΘ®1Θ©Νς≥Χ÷–Χζ‘”÷ ÷ς“Σ «Ά®ΙΐΉΣΜ·ΈΣ«β―θΜ·Χζ≥ΝΒμΒΡΖΫ Ϋ≥ΐ»ΞΘ§Υυ“‘Φ”»κœθΥαΒΡΡΩΒΡ «ΫΪ―«ΧζάκΉ”―θΜ·ΈΣΧζάκΉ”Θ§“‘±ψ”Ύ≥ΝΒμ≥ΐ‘”ΓΘ≥ΐœθΥαΆβΘ§ΜΙΩ…“‘ Ι”Ο¬Χ…Ϊ―θΜ·ΦΝΘΚΙΐ―θΜ·«βΘ§“‘±Θ÷Λ≤Μ“ΐ»κ‘”÷ ΓΘ”Ο¬»Υ°―θΜ·“≤ «“Μ÷÷Ω…––ΒΡ―Γ‘ώΘ§“ρΈΣ±ΨΝς≥Χ÷–¥φ‘ΎΒΡ“θάκΉ”ΨΆ «¬»άκΉ”ΓΘ
Θ®2Θ©ΗυΨίΥ°Ρύ÷–ΒΡ≥…Ζ÷Θ§“ΜΑψΕΰ―θΜ·Ιη≤Μ»ή”ΎΥαΘ§Υυ“‘≥ΝΒμA÷ς“Σ «Εΰ―θΜ·ΙηΓΘΤδ»ή”Ύ«βΖζΥαΒΡΖ¥”ΠΈΣΘΚSiO2 + 4HF === SiF4Γϋ+ 2H2OΓΘ
Θ®3Θ©Φ”»»Ω…“‘ Ι…ζ≥…ΒΡΫΚΧεΨέ≥ΝΘ§±ήΟβΫΚΧεΒΡ…ζ≥…Θ§“Ή”Ύ≥ΝΒμΒΡΖ÷άκΘΜpHΈΣ4ΓΪ5 ±Ca2+ΓΔMg2+≤Μ≥ΝΒμΘ§Fe3+ΓΔAl3+≥ΝΒμΘ§Υυ“‘≥ΝΒμBΈΣFe(OH)3ΓΔAl(OH)3ΓΘ
ΔρΘ®1Θ©HC2O4-ΒΡΥ°ΫβΖΫ≥Χ ΫΈΣΘΚHC2O4-+H2OH2C2O4+OH-Θ§HC2O4-ΒΡΥ°ΫβΤΫΚβ≥Θ ΐΈΣΘΚ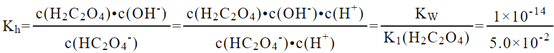 Θ§Υυ“‘Kh= 2ΓΝ10-13ΓΘ≤ίΥαΈΣΕΰ‘Σ»θΥαΘ§ΒγάκΙΐ≥ΧΖ÷≤ΫΫχ––Θ§≤ίΥαΒΡΒγάκΖΫ≥Χ ΫΈΣΘΚH2C2O4
Θ§Υυ“‘Kh= 2ΓΝ10-13ΓΘ≤ίΥαΈΣΕΰ‘Σ»θΥαΘ§ΒγάκΙΐ≥ΧΖ÷≤ΫΫχ––Θ§≤ίΥαΒΡΒγάκΖΫ≥Χ ΫΈΣΘΚH2C2O4![]() H++HC2O4-Θ§HC2O4-
H++HC2O4-Θ§HC2O4-![]() H++C2O42-ΘΜ≥ΘΈ¬œ¬ΫΪ0.2mol/LΒΡKOH»ή“Κ20mL”κ0.2mol/LΒΡ≤ίΥα»ή“Κ20mLΜλΚœΘ§Ζ¥”ΠΚσΒΟΒΫ≤ίΥα«βΦΊ»ή“ΚΘ§”…”Ύ≤ίΥα«βΗυάκΉ”ΒΡΒγάκΤΫΚβ≥Θ ΐΘ®K2Θ©¥σ”ΎΤδΥ°ΫβΤΫΚβ≥Θ ΐΘ®KhΘ©Θ§Υυ“‘Βγάκ’Φ÷ςΒΦΒΊΈΜΘ§»ή“Κœ‘Υα–‘ΓΘ
H++C2O42-ΘΜ≥ΘΈ¬œ¬ΫΪ0.2mol/LΒΡKOH»ή“Κ20mL”κ0.2mol/LΒΡ≤ίΥα»ή“Κ20mLΜλΚœΘ§Ζ¥”ΠΚσΒΟΒΫ≤ίΥα«βΦΊ»ή“ΚΘ§”…”Ύ≤ίΥα«βΗυάκΉ”ΒΡΒγάκΤΫΚβ≥Θ ΐΘ®K2Θ©¥σ”ΎΤδΥ°ΫβΤΫΚβ≥Θ ΐΘ®KhΘ©Θ§Υυ“‘Βγάκ’Φ÷ςΒΦΒΊΈΜΘ§»ή“Κœ‘Υα–‘ΓΘ
Θ®2Θ©ΧΦΥαΗΤ»ή“Κ÷–ΗΤάκΉ”≈®Ε»=![]() =5ΓΝ10-5mol/LΘ§Εΰ’ΏΜλΚœΚσΘ§ΗΤάκΉ”≈®Ε»=
=5ΓΝ10-5mol/LΘ§Εΰ’ΏΜλΚœΚσΘ§ΗΤάκΉ”≈®Ε»=![]() ΓΝ10-5mol/LΘ§≤ίΥαΦΊΒΡ≈®Ε»ΈΣ
ΓΝ10-5mol/LΘ§≤ίΥαΦΊΒΡ≈®Ε»ΈΣ![]() ΓΝ10-4mol/LΘ§ΜλΚœΚσc(Ca2+)c(C2O42-)=
ΓΝ10-4mol/LΘ§ΜλΚœΚσc(Ca2+)c(C2O42-)=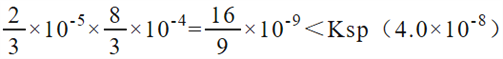 Θ§Ι Έό≥ΝΒμΓΘΧΦΥαΗΤΉΣΜ·ΈΣ≤ίΥαΗΤΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚCaCO3(s) + C2O42- (aq)
Θ§Ι Έό≥ΝΒμΓΘΧΦΥαΗΤΉΣΜ·ΈΣ≤ίΥαΗΤΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚCaCO3(s) + C2O42- (aq) ![]() CaC2O4(s) + CO32- (aq)Θ§Υυ“‘ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣΘΚ
CaC2O4(s) + CO32- (aq)Θ§Υυ“‘ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣΘΚ![]() ΓΘ
ΓΘ
Θ®3Θ©«β―θΜ·ΗΤΚΆ≤ίΥαΒ»Έο÷ ΒΡΝΩΖ¥”Π…ζ≥…≤ίΥαΗΤ≥ΝΒμΚΆΥ°Θ§Υυ“‘0.005mol/LΒΡ«β―θΜ·ΗΤ»ή“Κ20mL”κ0.0012mol/LΒΡ≤ίΥα»ή“Κ20mLΜλΚœΘ§ΒΟΒΫΒΡ «(0.005Θ≠0.0012)Γ¬2=0.0019mol/LΒΡ«β―θΜ·ΗΤ»ή“ΚΘ§Τδ÷–«β―θΗυάκΉ”ΒΡ≈®Ε»ΈΣ0.0038mol/LΓΘΥυ“‘90Γφœ¬ΒΡ«βάκΉ”ΈΣ38ΓΝ10-14Γ¬0.0038=1ΓΝ10-10mol/LΘ§Υυ“‘pH=10ΓΘ

ΓΨΧβΡΩΓΩ¥σΤχ÷–ΒΡ≤ΩΖ÷Ββ‘¥”Ύ![]() Ε‘ΚΘΥ°÷–
Ε‘ΚΘΥ°÷–![]() ΒΡ―θΜ·ΓΘΤδΩΤ―ß–ΓΉιΫχ––
ΒΡ―θΜ·ΓΘΤδΩΤ―ß–ΓΉιΫχ––![]() ”κΚ§
”κΚ§![]() »ή“ΚΖ¥”ΠΒΡœύΙΊ―–ΨΩΘΚ
»ή“ΚΖ¥”ΠΒΡœύΙΊ―–ΨΩΘΚ
Θ®1Θ©![]() ΫΪ
ΫΪ![]() ―θΜ·…ζ≥…
―θΜ·…ζ≥…![]() ΒΡΙΐ≥Χ”…3≤ΫΖ¥”ΠΉι≥…ΘΚ
ΒΡΙΐ≥Χ”…3≤ΫΖ¥”ΠΉι≥…ΘΚ
ΔΌ![]()
![]()
ΔΎ![]()
![]()
Δέ![]()
![]()
”Ο»»Μ·―ßΖΫ≥Χ Ϋ±μ Ψ![]() ―θΜ·
―θΜ·![]() …ζ≥…
…ζ≥…![]() ΒΡΖ¥”Π______ΓΘ
ΒΡΖ¥”Π______ΓΘ
Θ®2Θ©![]() ‘ΎΥ°÷–“ΉΖ÷ΫβΘ§“ΜΕ®ΧθΦΰœ¬Θ§
‘ΎΥ°÷–“ΉΖ÷ΫβΘ§“ΜΕ®ΧθΦΰœ¬Θ§ ![]() ΒΡ≈®Ε»Φθ…Ό“ΜΑκ ±Υυ–ηΒΡ ±ΦδΘ®tΘ©»γœ¬±μΥυ ΨΓΘ“―÷ΣΘΚ
ΒΡ≈®Ε»Φθ…Ό“ΜΑκ ±Υυ–ηΒΡ ±ΦδΘ®tΘ©»γœ¬±μΥυ ΨΓΘ“―÷ΣΘΚ ![]() ΒΡΤπ Φ≈®Ε»ΈΣ
ΒΡΤπ Φ≈®Ε»ΈΣ![]()
pH t/min T/Γφ | 3Θ°0 | 4Θ°0 | 5Θ°0 | 6Θ°0 |
20 | 301 | 231 | 169 | 58 |
30 | 158 | 108 | 48 | 15 |
50 | 31 | 26 | 15 | 7 |
ΔΌ![]() ‘ω¥σΡήΦ”ΥΌ
‘ω¥σΡήΦ”ΥΌ![]() Ζ÷ΫβΘ§±μΟςΕ‘
Ζ÷ΫβΘ§±μΟςΕ‘![]() Ζ÷ΫβΤπ¥ΏΜ·Ής”ΟΒΡ «___________ΓΘ
Ζ÷ΫβΤπ¥ΏΜ·Ής”ΟΒΡ «___________ΓΘ
ΔΎΗυΨί±μ÷– ΐΨίΘ§ΆΤ≤β![]() ‘Ύœ¬Ν–ΧθΦΰœ¬Ζ÷ΫβΥΌ¬ “ά¥Έ‘ω¥σΒΡΥ≥–ρΈΣ__________Θ®ΧνΉ÷ΡΗ¥ζΚ≈Θ©
‘Ύœ¬Ν–ΧθΦΰœ¬Ζ÷ΫβΥΌ¬ “ά¥Έ‘ω¥σΒΡΥ≥–ρΈΣ__________Θ®ΧνΉ÷ΡΗ¥ζΚ≈Θ©
a. 40ΓφΓΔ![]() b. 10ΓφΓΔ
b. 10ΓφΓΔ![]() c. 30ΓφΓΔ
c. 30ΓφΓΔ![]()
Θ®3Θ©![]() ‘ΎKI»ή“Κ÷–¥φ‘Ύœ¬Ν–ΤΫΚβΘΚ
‘ΎKI»ή“Κ÷–¥φ‘Ύœ¬Ν–ΤΫΚβΘΚ![]() ΓΘ≤βΒΟ≤ΜΆ§Έ¬Ε»œ¬ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ»γΆΦΥυ ΨΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «__________ΓΘ
ΓΘ≤βΒΟ≤ΜΆ§Έ¬Ε»œ¬ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ»γΆΦΥυ ΨΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «__________ΓΘ

A. Ζ¥”Π![]() ΒΡ
ΒΡ![]()
B. άϊ”ΟΗΟΖ¥”ΠΩ…“‘≥ΐ»ΞΝρΖέ÷–…ΌΝΩΒΡΒβΒΞ÷
C. ‘Ύ…œ ωΤΫΚβΧεœΒ÷–Φ”»κ±ΫΘ§ΤΫΚβ≤Μ“ΤΕ·
D. 25Γφ ±Θ§‘Ύ…œ ωΤΫΚβΧεœΒ÷–Φ”»κ…ΌΝΩ![]() ΙΧΧεΘ§ΤΫΚβ≥Θ ΐK–Γ”Ύ680
ΙΧΧεΘ§ΤΫΚβ≥Θ ΐK–Γ”Ύ680
Θ®4Θ©ΫΪ![]() »ή”Ύ
»ή”Ύ![]() ÷–Θ§ΒΟΒΫΉœΚλ…ΪΒΡ»ή“ΚΘ§‘ΌΦ”»κ“ΜΕ®≈®Ε»ΒΡ
÷–Θ§ΒΟΒΫΉœΚλ…ΪΒΡ»ή“ΚΘ§‘ΌΦ”»κ“ΜΕ®≈®Ε»ΒΡ![]() »ή“ΚΘ§œ÷œσ»γΆΦΥυ ΨΘΚ
»ή“ΚΘ§œ÷œσ»γΆΦΥυ ΨΘΚ

ΔΌ…œ≤ψ»ή“Κ÷–Κ§Ββ‘ΣΥΊΒΡΈΔΝΘ”–_______________Θ®”ΟΜ·―ßΖϊΚ≈±μ ΨΘ©ΓΘ
ΔΎ”… Β―ιœ÷œσΩ…ΆΤ≤βΙΊ”Ύ![]() »ήΫβ–‘ΒΡΫα¬έ «______________ΓΘ
»ήΫβ–‘ΒΡΫα¬έ «______________ΓΘ
ΓΨΧβΡΩΓΩογœΒΈΣ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎΔσBΉεΓΔ‘≠Ή”–ρ ΐΈΣ57~71ΒΡ‘ΣΥΊΓΘ
Θ®1Θ©οα(Dy)ΒΡΜυΧ§‘≠Ή”ΒγΉ”≈≈≤Φ ΫΈΣ[Xe]4f106s2Θ§Μ≠≥ωοα(Dy)‘≠Ή”ΆβΈßΒγΉ”≈≈≤ΦΆΦΘΚ___________ΓΘ
Θ®2Θ©ΗΏΈ¬≥§ΒΦ≤ΡΝœογ±ΒΆ≠―θΜ·Έο÷–Κ§”–Cu3+Θ§ΜυΧ§ ±Cu3+ ΒΡΒγΉ”≈≈≤Φ ΫΈΣ________________ΓΘ
Θ®3Θ©Ιέ≤λœ¬ΟφΥΡ÷÷ογœΒ‘ΣΥΊΒΡΒγάκΡή ΐΨίΘ§≈–ΕœΉν”–Ω…Ρήœ‘ Ψ+3 ΦέΒΡ‘ΣΥΊ «___________(Χν‘ΣΥΊΟϊ≥Τ)ΓΘΦΗ÷÷ογœΒ‘ΣΥΊΒΡΒγάκΡή(ΒΞΈΜΘΚkJ mol-1)
‘ΣΥΊ | I1 | I2 | I3 | I4 |
Yb(ονΘ© | 604 | 1217 | 4494 | 5014 |
Lu(οεΘ© | 532 | 1390 | 4111 | 4987 |
La(ογΘ© | 538 | 1067 | 1850 | 5419 |
Ce(νφΘ© | 527 | 1047 | 1949 | 3547 |
Θ®4Θ©‘ΣΥΊνφ(Ce)Ω…“‘–Έ≥…≈δΚœΈο(NH4)2[Ce(NO3)6]ΓΘ
ΔΌΉι≥…≈δΚœΈοΒΡΥΡ÷÷‘ΣΥΊΘ§ΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________________(”Ο‘ΣΥΊΖϊΚ≈±μ Ψ)ΓΘ
ΔΎ–¥≥ωΒΣΒΡΉνΦρΒΞΤχΧ§«βΜ·ΈοΥ°»ή“Κ÷–¥φ‘ΎΒΡ«βΦϋΘΚ__________________(»Έ–¥“Μ÷÷)ΓΘ
Δέ‘ΣΥΊAl “≤”–άύΥΤ≥…Φϋ«ιΩωΘ§ΤχΧ§¬»Μ·¬ΝΖ÷Ή”±μ ΨΈΣ(AlCl3)2Θ§Ζ÷Ή”÷–Al ‘≠Ή”‘”Μ·ΖΫ ΫΈΣ_____________Θ§Ζ÷Ή”÷–ΥυΚ§Μ·―ßΦϋάύ–Ά”–______________(ΧνΉ÷ΡΗ)ΓΘ
a.άκΉ”Φϋ b.ΦΪ–‘Φϋ C.Ζ«ΦΪ–‘Φϋ d.≈δΈΜΦϋ
Θ®5Θ©PrO2(Εΰ―θΜ·οη)ΒΡΨßΧεΫαΙΙ”κCaF2œύΥΤΘ§ΨßΑϊ÷–οη‘≠Ή”ΈΜ”ΎΟφ–ΡΚΆΕΞΒψΘ§‘ρPrO2(Εΰ―θΜ·οη)ΒΡΨßΑϊ÷–”–________Ηω―θ‘≠Ή”ΘΜ“―÷ΣΨßΑϊ≤Έ ΐΈΣa pmΘ§ΟήΕ»ΈΣΠ― gΓΛ cm-3Θ§NA=_____________ (”ΟΚ§aΓΔΠ―ΒΡ¥ζ ΐ Ϋ±μ Ψ)ΓΘ