��Ŀ����
����Ŀ����������NOCl�����ںϳ������ȡ�������Cl2��NO�ڳ��³�ѹ�ºϳɣ������۵�Ϊ-64.5�棬�е�Ϊ-5.5�棬�������ǻ�ɫ���ж����壬��ˮ��ˮ�⡣ �밴Ҫ��ش�����������⣺
(1)������м��ϡ�����ַ�Ӧ�Ʊ�NO�����ӷ���ʽΪ��______________________��
(2)�Ʊ�NOCl��װ������ͼ��ʾ������˳��Ϊ:a��_________________________(�������������ҷ�����Сд��ĸ��ʾ)��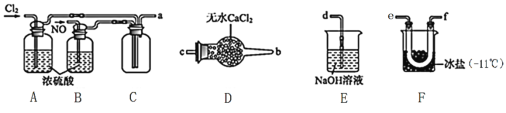
��װ��A��B�����Ǣٸ���NO��Cl2����___________________________________________��
��װ��D��������______________________________________��
��װ��E��NOCl������Ӧ�Ļ�ѧ����ʽΪ________________��
(3)��ҵ����������NOβ�����������ж��֣����м�ӵ绯ѧ������ԭ����ͼ��ʾ��

�ù����������ĵ缫��ӦʽΪ��__________________________________________��
���𰸡�3Fe+8H++2NO3��=3Fe2++2NO��+4H2O e��f��c��b��d��f��e��c��b��d �۲����ݵ����������� ��ֹE��ˮ��������F������NOCl��ˮ�� NOCl+2NaOH=NaCl+NaNO2+H2O 2HSO3��+2e��+2H+= S2O42��+ 2H2O
��������
��1��������м��ϡ���ᷴӦ��������������һ��������ˮ��
��2����������NO�������װ��F�з�����Ӧ���ڱ����������ռ�NOCl��������NO�Լ�NOCl�������ŷŵ������У�������������Һ���գ�ע��NOCl��ˮ��ˮ�⡣
��3���ɼ�ӵ绯ѧ��ʾ��ͼ��֪��������HSO3���ŵ�����S2O42����S2O42�����������н�NO��ԭΪN2��
��1��������м��ϡ���ᷴӦ��������������һ��������ˮ����Ӧ�����ӷ���ʽΪ3Fe+8H++2NO3��=3Fe2++2NO��+4H2O���ʴ�Ϊ��3Fe+8H++2NO3��=3Fe2++2NO��+4H2O��
��2����������NO�������װ��F�з�����Ӧ���ڱ����������ռ�NOCl��������NO�Լ�NOCl�������ŷŵ������У�������������Һ���գ�NOCl��ˮ��ˮ�⣬�����ռ�װ�ú�β������װ��֮�����һ������װ�ã��ʴ�Ϊ��e��f����f��e����c��b��d��
��װ��A��B�����dz�����NO��Cl2�⣬��һ��������ͨ���۲����ݵ�����������ٿ��Ʒ�Ӧ�ķ������ʴ�Ϊ���۲����ݵ����������٣�
��NOCl��ˮ��ˮ�⣬װ��D���Ȼ����������������ˮ��������ֹE��ˮ�������뷴Ӧ��F�У�����NOCl��ˮ�⣬�ʴ�Ϊ����ֹE��ˮ��������F������NOCl��ˮ�⣻
��װ��E��Ŀ������β������ֹ��Ⱦ������NOCl������������Һ��Ӧ����NaCl��NaNO2��H2O����Ӧ�Ļ�ѧ����ʽΪNOCl+2NaOH=NaCl+NaNO2+H2O���ʴ�Ϊ��NOCl+2NaOH=NaCl+NaNO2+H2O��
��3���ɼ�ӵ绯ѧ��ʾ��ͼ��֪��������HSO3���ŵ�����S2O42����S2O42�����������н�NO��ԭΪN2�������ĵ缫��ӦʽΪ2HSO3��+2e��+2H+= S2O42��+ 2H2O���ʴ�Ϊ��2HSO3��+2e��+2H+= S2O42��+ 2H2O��

����Ŀ��t��ʱ����һ�����Ϊ2L�ܱ������м��뷴Ӧ��A��B���������·�Ӧ��A(s)+2B(g)![]() 3C(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
3C(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
���� | ��ʼ | 2���� | 4���� | 6���� |
A | 2 mol | 1.2 mol | ||
B | 6 mol | 3.0 mol | ||
C | 0 mol | x mol | 4.5 mol |
A. ǰ2�����ڣ�A�ķ�Ӧ����Ϊ0.2molL-1min-1
B. ����x��ֵΪ3.6
C. 4����ʱ����Ӧ�ﵽƽ��״̬����ʱ�����淴Ӧ�����ʶ�Ϊ0
D. �����¶ȣ������淴Ӧ�����ʶ�������




