��Ŀ����
��һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�������ʵ�飬
ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������
(1)����ǰͨ�������Ŀ���� ��
��������Ϊ ��
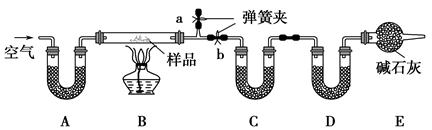
(2)װ��A��C��D��ʢ�ŵ��Լ��ֱ�Ϊ��A ��
C ��D ��
(3)����Aװ�û���ʢ��NaOH��Һ��ϴ��ƿ�����õ�NaCl�ĺ����� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)����B�з�Ӧ���Ҳ���ˮ������������ⶨ�����NaHCO3�ĺ����� ������ȥEװ�ã�����Na2CO3��10H2O�ĺ����� ��
(4)����Ʒ����Ϊw g����Ӧ��C��D���ӵ������ֱ�Ϊm1 g��m2 g���ɴ˿�֪�������NaHCO3����������Ϊ (�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������

(1)����ǰͨ�������Ŀ���� ��
��������Ϊ ��
(2)װ��A��C��D��ʢ�ŵ��Լ��ֱ�Ϊ��A ��
C ��D ��
(3)����Aװ�û���ʢ��NaOH��Һ��ϴ��ƿ�����õ�NaCl�ĺ����� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)����B�з�Ӧ���Ҳ���ˮ������������ⶨ�����NaHCO3�ĺ����� ������ȥEװ�ã�����Na2CO3��10H2O�ĺ����� ��
(4)����Ʒ����Ϊw g����Ӧ��C��D���ӵ������ֱ�Ϊm1 g��m2 g���ɴ˿�֪�������NaHCO3����������Ϊ (�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
(1)��ȥװ���е�ˮ�����Ͷ�����̼���ر�b����a������ͨ�������ֱ��a�������Ŀ�������ʹ����ʯ��ˮ�����Ϊֹ
(2)��ʯ�ҡ���ˮ����ͭ(����ˮCaCl2��P2O5��)����ʯ��
(3)ƫ�͡���Ӱ�졡ƫ��
(4)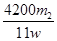 %
%
(2)��ʯ�ҡ���ˮ����ͭ(����ˮCaCl2��P2O5��)����ʯ��
(3)ƫ�͡���Ӱ�졡ƫ��
(4)
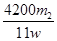 %
%(2)A��C��D����U�ιܣ�����ʢҺ���Լ���ֻ��ʢ�����Լ���A���Լ�����ͬʱ����CO2��ˮ��������A��ʢ���Ǽ�ʯ�ң�C��D�����ֱ�����ˮ������CO2����C����ʢ��ˮ����ͭ(����ˮCaCl2��P2O5��)��D����ʢ��ʯ�ҡ�
(3)��Aװ��ʢ��NaOH��Һֻ����CO2��������ˮ�������������в���ˮ������Na2CO3��10H2O��NaHCO3���������ڼ����л�������˲�õ�NaCl�ĺ�����ƫ�ͣ���B�з�Ӧ���Ҳ���ˮ������������ˮ������������С��Na2CO3��10H2O��NaHCO3���������ڼ����л��С������NaHCO3�������Ǹ���CO2���������м��㣬���Բ�õ�NaHCO3�ĺ�������Ӱ�죻����ȥEװ�ã���Dװ�ÿ����������������е�CO2��ʹ��NaHCO3�������ڼ����л����ʲ�õ�Na2CO3��10H2O�ĺ�����ƫ�͡�
(4)NaHCO3������������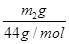 ��2��84 g��mol��1��w g��100%��
��2��84 g��mol��1��w g��100%��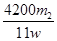 %��
%��
(3)��Aװ��ʢ��NaOH��Һֻ����CO2��������ˮ�������������в���ˮ������Na2CO3��10H2O��NaHCO3���������ڼ����л�������˲�õ�NaCl�ĺ�����ƫ�ͣ���B�з�Ӧ���Ҳ���ˮ������������ˮ������������С��Na2CO3��10H2O��NaHCO3���������ڼ����л��С������NaHCO3�������Ǹ���CO2���������м��㣬���Բ�õ�NaHCO3�ĺ�������Ӱ�죻����ȥEװ�ã���Dװ�ÿ����������������е�CO2��ʹ��NaHCO3�������ڼ����л����ʲ�õ�Na2CO3��10H2O�ĺ�����ƫ�͡�
(4)NaHCO3������������
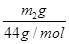 ��2��84 g��mol��1��w g��100%��
��2��84 g��mol��1��w g��100%��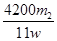 %��
%��
��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ