��Ŀ����
15����3L�ܱ������У�����1mol N2��3 mol H2����һ�������·�����Ӧ��20s��ﵽ��Ӧ�ȣ���û�����������ʵ���Ϊ3.4mol�����м�������ȷ���ǣ�������| A�� | 20s�������n��H2����n��N2����n��NH3��=1��2��3 | |
| B�� | N2��ת����Ϊ30% | |
| C�� | 20s��H2��ƽ����Ӧ����Ϊ1.5mol•L-1•s-1 | |
| D�� | NH3���������Ϊ25% |
���� ��3L�ܱ������У�����1mol N2��3 mol H2����һ�������·�����Ӧ��20s��ﵽ��Ӧ�ȣ���Ӧ�ﵽƽ��״̬���跴Ӧ�ĵ������ʵ���Ϊx����ϻ�ѧƽ������ʽ��ʽ���㣬��û�����������ʵ���Ϊ3.4mol��
N2+3H2=2NH3
��ʼ����mol�� 1 3 0
�仯����mol�� x 3x 2x
ƽ������mol��1-x 3-3x 2x
1-x+3-3x+2x=3.4
x=0.3mol�����ݼ���������ѡ�������
��� �⣺�跴Ӧ�ĵ������ʵ���Ϊx��
N2+3H2=2NH3
��ʼ����mol�� 1 3 0
�仯����mol�� x 3x 2x
ƽ������mol�� 1-x 3-3x 2x
��û�����������ʵ���Ϊ3.4mol����1-x+3-3x+2x=3.4�����x=0.3mol��
A��20s�������n��H2����n��N2����n��NH3��=��3-3��0.3������1-0��3����2��0.3=��21��7��6����A����
B��N2��ת����=$\frac{0.3mol}{1mol}$��100%=30%����B��ȷ��
C��20s��H2��ƽ����Ӧ����=$\frac{\frac{3��0.3mol}{3L}}{20s}$=0.015mol•L-1•s-1����C����
D��NH3���������Ϊ=$\frac{2x}{4-2x}$��100%=$\frac{2��0.3}{4-2��0.3}$��100%=17.6%����D����
��ѡB��
���� ���⿼���˻�ѧƽ��ļ���Ӧ�ã���Ҫ������ʽ�����������Ӧ���ʡ�Ũ�ȡ������������ļ���Ӧ�ã����ջ����ǹؼ�����Ŀ�ϼ�

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д�| A�� | 40.7 | B�� | 46 | C�� | 36 | D�� | 44 |
������ԭ�ӹ��ɵĹ��ۻ���������еĻ�ѧ�����Ǽ��Լ�
�����ֲ�ͬ�ǽ���Ԫ��ԭ�Ӽ��γɵĻ�ѧ�����Ǽ��Լ�
�ۺ��зǼ��Լ��Ļ�����һ���ǹ��ۻ����ֻҪ�����ӻ�������۵�ͱȹ��ۻ�������۵��
����ʧȥ���ӵ�ԭ�ӣ����γ�������
�ݵ��ʷ����в����ڻ�ѧ����������ķ����вŴ��ڻ�ѧ��
�����ӻ�������һ���������Ӽ���
| A�� | ֻ�Тڢ� | B�� | ֻ�Т٢� | C�� | ֻ�Т� | D�� | ֻ�Т٢ܢ� |
| A�� | �ڢۢ� | B�� | �ۢܢ� | C�� | �ڢܢ� | D�� | �ܢ� |
| A�� | ����������Ӧˮ��������ԣ�H2SiO3��H3PO4��H2SO4 | |
| B�� | ����ˮ�ķ�Ӧ���ʣ�K��Na��Mg | |
| C�� | ���ȶ��ԣ�HF��HCl��H2S | |
| D�� | ���Ӱ뾶��Cl-��F-��Na+ |
| A�� | 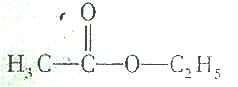 | B�� | 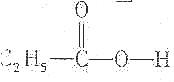 | ||
| C�� | CH2�TCH-O-CH2CH3 | D�� | 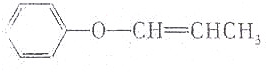 |
 ����������ϩ���ʹμ�֮�����ͨ�������������������е����ṹʱ�������е���ϩ�����Ǽ�������ΪC
����������ϩ���ʹμ�֮�����ͨ�������������������е����ṹʱ�������е���ϩ�����Ǽ�������ΪC