��Ŀ����
5����Fe�ۡ�Cu�ۡ�FeCl3��Һ��FeCl2��Һ��CuCl2��Һ�������ij�����г�ַ�Ӧ���ٶ����������뷴Ӧ�������ж������������Һ�д��ڵĽ������Ӻͽ������ʣ���1����������ʣ�࣬�������в�������Fe3+��Cu2+��
��2�����Ȼ�ͭ��ʣ�࣬�������л�������Fe3+��Fe2+��Cu��Fe2+��
��3�����Ȼ������Ȼ�ͭ����ʣ�࣬�������в�������Fe��Cu��һ����Fe2+��
��4�����Ȼ�����ʣ�࣮�������в�������Fe��Cu��
��5�������Ϸ�Ӧ��֪���ӵ�������ǿ��˳��ΪFe3+��Cu2��Fe2+��
���� �ڽ������˳���У�λ����ǰ�Ľ����ܽ�λ�����Ľ�����������Һ���û���������װ���Ȼ�����Һ���ձ��У�����һ������Cu��Fe�Ļ�Ϸ�ĩ��������ͭ�������Ȼ�����Һ��Ӧ�������ԣ�FeCl3��CuCl2��FeCl2����ԭ��Fe��Cu��Fe��ʣ�࣬��Cuû�вμӷ�Ӧ����Һ�в�����Fe3+��Cu2+���Դ˽����⣮
��� �⣺�����ԣ�FeCl3��CuCl2��FeCl2����ԭ��Fe��Cu��
��1����Ӧ������ʣ�࣬����Fe+2FeCl3=3FeCl2��Fe+CuCl2=Cu+FeCl2��Fe3+��Cu2+�����ܴ��ڣ��ʴ�Ϊ��Fe3+��Cu2+��
��2����CuCl2��ʣ�࣬�����Ȼ�ͭ��������������������Fe�������п�����Fe3+��Fe2+��Cu��Fe2+���ʴ�Ϊ��Fe3+��Fe2+��Cu��Fe2+��
��3��FeCl3��CuCl2����ʣ�࣬��Fe��Cu����ȫ��Ӧ�����ܴ��ڣ�������һ������Fe2+���ʴ�Ϊ��Fe��Cu��Fe2+��
��4����FeCl3��ʣ�࣬����Fe+2FeCl3=3FeCl2��Cu+2FeCl3=CuCl2+2FeCl2��Fe��Cu�����ܴ��ڣ��ʴ�Ϊ��Fe��Cu��
��5���ɷ�ӦFe+2FeCl3=3FeCl2��Fe+CuCl2=Cu+FeCl2��������ǿ��˳��ΪFe3+��Cu2��Fe2+���ʴ�Ϊ��Fe3+��Cu2��Fe2+��
���� ���⿼��������ԭ��Ӧ�Լ����ĵ��ʻ���������ʣ���Ŀ�ѶȲ�����ؼ��ǰ������ʵ������ԡ���ԭ��ǿ���жϷ�Ӧ���Ⱥ�˳��

 ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�| A�� |  | B�� |  | C�� |  | D�� |  |
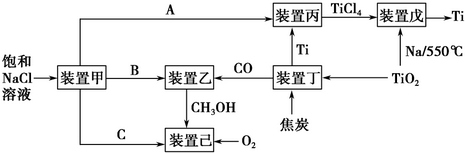
��ش��������⣺
��1��װ�ü�Ϊ���أ����Ե缫��������ͼʾת����ϵ��֪��AΪCl2���ѧʽ����������ӦʽΪ2H++2e-=H2����
��2��װ�ñ��ķ�Ӧ��ΪTi����װ�����������ΪTi��������װ���ڸ����������в���ì�ܣ�ԭ���ǽ���װ�ñ���Ti���е����ʽ϶࣬��װ�����г�����Ti��Ϊ����
װ������з�Ӧʱ��Ҫ�Ļ���ΪC������ĸ��ţ���
A��HCl�����Χ�� B��������Χ�� C�������Χ�� D��ˮ��
��3��װ�����з������ǹ�ҵ�ϳɼ״��ķ�Ӧ��CO��g��+2H2��g��=CH3OH��g����H��0��
�ٸ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K�����±���
| �¶�/�� | 250 | 350 |
| K | 2.041 | x |
A��0 B��0.012 C��32.081 D��100
����װ����Ϊ�ݻ��̶����ܱ���������ͬʱ��θ����ʵ�Ũ�����±���
| c��CO��/mol•L-1 | c��H2��/mol•L-1 | c��CH3OH��/mol•L-1 | |
| 0min | 0.8mol•L-1 | 1.6mol•L-1 | 0 |
| 2min | 0.6mol•L-1 | y | 0.2mol•L-1 |
| 4min | 0.3mol•L-1 | 0.6mol•L-1 | 0.5mol•L-1 |
| 6min | 0.3mol•L-1 | 0.6mol•L-1 | 0.5mol•L-1 |
Aʹ�ô��� B�����¶�C����H2��Ũ��
��4��װ�ü����Կ���ȼ�ϵ�أ���ȼ�ϵ�صĸ�����ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
| A�� | 20s�������n��H2����n��N2����n��NH3��=1��2��3 | |
| B�� | N2��ת����Ϊ30% | |
| C�� | 20s��H2��ƽ����Ӧ����Ϊ1.5mol•L-1•s-1 | |
| D�� | NH3���������Ϊ25% |
 ����������Ϊ���Ӿ��壮
����������Ϊ���Ӿ��壮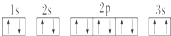 ����ͬѧ�����ĵ����Ų�ͼΥ��������ԭ����
����ͬѧ�����ĵ����Ų�ͼΥ��������ԭ���� ��1����9��������NH2-����-NH2����Br-����OH-����-NO2����-OH����NO2��
��1����9��������NH2-����-NH2����Br-����OH-����-NO2����-OH����NO2��