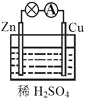
��Ŀ����
����Ŀ���������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ��(��ͼ��ʾ)��
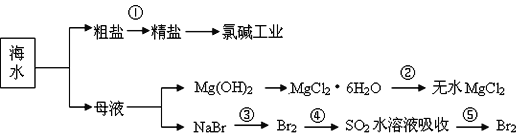
�����й�˵����ȷ����
A.�������г�ȥ�����е�![]() ��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ�� Na2CO3��Һ��BaCl2��Һ��NaOH��Һ�����������������pH
��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ�� Na2CO3��Һ��BaCl2��Һ��NaOH��Һ�����������������pH
B.MgSO4��7H2O�ڿ����м��ȿ��Ƶ���ˮMgSO4�ķ�������������ơ�
C.�ӵ���������������Ŀ����Ϊ��Ũ������Br2
D.�ڵ��ۢܢ�������Ԫ�ؾ�������
���𰸡�C
��������
A��ѡ���е��Լ�����˳���У��������������ȥ��������ҩƷ˳��Ϊ��BaCl2��Һ��NaOH��Һ��Na2CO3��Һ�����˺�����ᣬ��A����
B��MgSO4��7H2O�ڿ����м��ȿ��Ƶ���ˮMgSO4�����ڢ��е�MgCl26H2O��ˮ������þ������ˮ������������þ���������Ȼ�����������ˮ��������þ����ˮ�⣬����ȷ�����һ������B����
C���ڢ۲��������ӱ�����Ϊ�嵥�ʣ��ڢܲ����嵥�ʱ���ԭΪ�����ӣ��ڢݲ��������ӱ�����Ϊ�嵥�ʣ����̵�Ŀ����Ũ������C��ȷ��
D���ڢ۲��������ӱ�����Ϊ�嵥�ʣ��ڢܲ����嵥�ʱ���ԭΪ�����ӣ��ڢݲ��������ӱ�����Ϊ�嵥�ʣ���D����
�ʴ�ΪC��

����Ŀ����һ���¶��£�10mL0.40mol/L H2O2�������ֽ⡣��ͬʱ�̲ⶨ����O2�������������Ϊ��״�������±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ���ǣ���Һ����仯���Բ��ƣ�
A��0~6min��ƽ����Ӧ���ʣ�v��H2O2��![]()
![]() mol/(L��min)
mol/(L��min)
B��6~10min��ƽ����Ӧ���ʣ�v��H2O2����![]() mol/(L��min)
mol/(L��min)
C����Ӧ��6minʱ��c��H2O2��=0.3mol/L
D����Ӧ��6minʱ��H2O2�ֽ���50%
����Ŀ���л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ �� �� �� | �� �� �� ʵ �� �� �� |
��1����ȡA 9.0g������ʹ�������������ܶ�����ͬ������H2��45���� | ��ͨ��������գ� ��1��A����Է�������Ϊ��____�� |
��2������9.0gA��������O2���ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4g��13.2g�� | ��2��A������ʽΪ��_______�� |
��3����ȡA 9.0g����������NaHCO3��ĩ��Ӧ������2.24LCO2����״�������������������Ʒ�Ӧ������2.24LH2����״������ | ��3�����ṹ��ʽ��ʾA�к��еĹ����ţ�___________�� |
��4��A�ĺ˴Ź�����������ͼ��
| ��4��A�к���________����ԭ�ӡ� |
��5������������A���ṹ��ʽ_____________�� | |