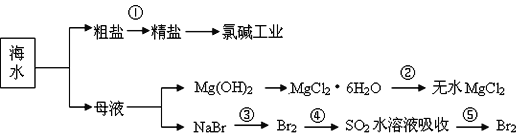
��Ŀ����
����Ŀ���л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ �� �� �� | �� �� �� ʵ �� �� �� |
��1����ȡA 9.0g������ʹ�������������ܶ�����ͬ������H2��45���� | ��ͨ��������գ� ��1��A����Է�������Ϊ��____�� |
��2������9.0gA��������O2���ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4g��13.2g�� | ��2��A������ʽΪ��_______�� |
��3����ȡA 9.0g����������NaHCO3��ĩ��Ӧ������2.24LCO2����״�������������������Ʒ�Ӧ������2.24LH2����״������ | ��3�����ṹ��ʽ��ʾA�к��еĹ����ţ�___________�� |
��4��A�ĺ˴Ź�����������ͼ��
| ��4��A�к���________����ԭ�ӡ� |
��5������������A���ṹ��ʽ_____________�� | |
���𰸡�90 C3H6O3 -COOH��-OH 4 ![]()
��������
��1����������ܶ�ȷ����Է���������
��2�������л���ȼ��ȷ������ʽ��
��3��������������ȷ�������ŵĴ��ڣ�
��4�����ݺ˴Ź�������ȷ����ԭ�����ࣻ
��5���ۺ�������Ϣȷ���л���ṹ��ʽ��
��1����������ܶȿ�֪���л����Ħ������Ϊ45��2g/mol=90g/mol��Ħ��������g/molΪ��λʱ������ֵ�ϵ�������Է�������������Է�������Ϊ90��
��2��9.0g�л�������ʵ���Ϊ![]() ����Ũ��������13.2g��֪��ԭ�ӵ����ʵ���Ϊ
����Ũ��������13.2g��֪��ԭ�ӵ����ʵ���Ϊ![]() �����ݼ�ʯ������13.2g��֪̼�����ʵ���Ϊ
�����ݼ�ʯ������13.2g��֪̼�����ʵ���Ϊ![]() ������������ʵ���Ϊ
������������ʵ���Ϊ![]() ������л���ķ���ʽΪC3H6O3��
������л���ķ���ʽΪC3H6O3��
��3�����ڸ��л����ܹ���̼�����Ʒ�Ӧ����������̼�����Ը��л����к����Ȼ������Ȼ���ĿΪ1��������л��������������Ʒ�Ӧ������������0.1mol���л������0.1mol���������Ը��л��ﺬ���ǻ��������л��ﺬ�еĹ�����Ϊ�Ȼ����ǻ���
��4�����ݺ˴Ź�������ͼ������֪���л����к���4����ԭ�ӣ�
��5�������л���ķ���ʽ���������Լ��˴Ź�������֪���л���Ľṹ��ʽΪ![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����֪��KClO3��6HCl(Ũ)===KCl��3Cl2����3H2O.��ͼ��ʾ���������Լ��ֱ�����������е���Ӧλ�ã�ʵ��ʱ��Ũ������� KClO3 �����ϣ����ñ�����Ǻá��±�����ʵ������ó��Ľ�����ȫ��ȷ���� ( )
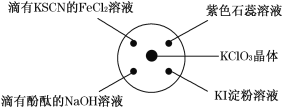
ѡ�� | ʵ������ | ���� |
A | ���� KSCN �� FeCl2 ��Һ���ɫ | Cl2 ���л�ԭ�� |
B | ���з�̪�� NaOH ��Һ��ɫ | Cl2 �������� |
C | ���� KI ��Һ�б���ɫ | Cl2 ���������� |
D | ʯ����Һ�ȱ�Ϊ��ɫ����ɫ | Cl2 ����Ư���� |
A. A B. B C. C D. D
����Ŀ���ȱ��ĺϳɹ��շ�ΪҺ�෨�����෨���֣�ʵ����ģ��Һ�෨��װ������ͼ�����мг�����������װ�ü�β������װ������ȥ��,�й����ʵ����������ʾ
���� | ��Է������� | �е�/(��) | �ܶ�/(g/mL) |
�� | 78 | 78 | 0.88 |
�ȱ� | 112.5 | 132.2 | 1.1 |
�ڶ��ȱ� | 147 | 180.4 | 1.3 |
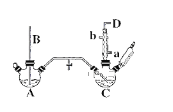
�ش��������⣺
��1��A��Ӧ��������ʵ���ҷ���ȡ������װ�����пյ���B��������_______________��
��2���Ѹ��������ͨ��װ��50.0mL���ﱽ��������м��������ƿC���Ʊ��ȱ���д���������Ʊ��ȱ��Ļ�ѧ����ʽ��_______________��
��3��C�ķ�Ӧ�¶Ȳ��˹��ߣ�ԭ��Ϊ���¶ȹ��ߣ���Ӧ�õ����ȱ�����_______________��D���ڵ���Ҫβ���ɷ���___________________��
��4���ᴿ�ֲ�Ʒ�������£�
![]()
���������м�����ˮCaCl2��Ŀ����_____________��
��5��ʵ�������յõ���Ʒ22.5mL������Ϊ______________��
��6�����෨���ȱ��ǽ��������Ȼ������������뱽���¶�Ϊ210�棬�Ͽ��ʹ�����CuCl2��FeCl3�������������ϣ������½����Ȼ���������Ӧ�����ȱ����䷴Ӧ����ʽΪ��_______________��