��Ŀ����
10��ijʵ���о�С����Ҵ������ᡢ���������Ļ�����з��롢���������������ʣ���������·�����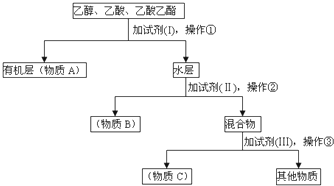
��1��ʵ������У������ٵ������Ƿ�Һ������ѡ�õ�װ����d�����ţ���
��2��A��B��C�DZ����յ����ʣ�����A���������ŵĽṹ��ʽΪ��-COOC-��A���п��ܺ���������B���B����C�������ʣ������Ƿ��д����ʣ���ǡ�����Լ��ǣ�Na��
��3��ʵ���������Լ��ֱ��ǣ�I����̼���ơ����е�Ũ�ȵ�����������Լ�II��ֱ�ӽ��в����ڣ�����B�п��ܺ��е������ǣ�ˮ��
���� �ɷ������̿�֪���Լ�IΪ����̼������Һ��������Ϊ��Һ���Լ�II�ɼ�CaO�ӣ������ڷ�����ܽ�Ļ��Һ�壬Ϊ�������Լ���������ת�������ᣬ��Ҫ�����ȶ��ġ����ӷ���ǿ�ᣬ���Լ�IIΪ���ᣬ�����۷����������������Һ�����뷽��Ϊ�����Դ������
��� �⣺�ɷ������̿�֪���Լ�IΪ����̼������Һ��������Ϊ��Һ���Լ�II�ɼ�CaO�ӣ������ڷ�����ܽ�Ļ��Һ�壬Ϊ�������Լ���������ת�������ᣬ��Ҫ�����ȶ��ġ����ӷ���ǿ�ᣬ���Լ�IIΪ���ᣬ�����۷����������������Һ�����뷽��Ϊ����
��1��������������֪�������ٵ������Ƿ�Һ��������ѡ�õ�װ����d���ʴ�Ϊ����Һ��d��
��2��������������֪��AΪ�������������������ŵĽṹ��ʽΪ-COOC-��A���п��ܺ���������B���Ҵ��������Լ�Na�ɼ��飬��������������B����֮������
�ʴ�Ϊ��-COOC-��B��Na��
��3��������������֪���Լ�IΪ����̼���ƣ��Լ���Ϊ�е�Ũ�ȵ����ᣬ�������Լ�II��ֱ�ӽ��в����ڣ�����B�п��ܺ��е�������ˮ���Լ�II�ɳ�ȥˮ��
�ʴ�Ϊ������̼���ƣ��е�Ũ�ȵ����ˮ��
���� ���⿼����������ᴿ��ʵ����ƣ�Ϊ��Ƶ���㣬�������л���֪ʶ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬���ջ����������̡����뷽���������ķ�Ӧ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | NaHSO4�ĵ��룺NaHSO4=Na++H++SO${\;}_{4}^{2-}$ | |
| B�� | �����ǵĽṹ��ʽ��C6H12O6 | |
| C�� | S2-�Ľṹʾ��ͼ��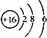 | |
| D�� | ������Ϊ94��������Ϊ144���У�Pu��ԭ�ӣ�${\;}_{92}^{144}$Pu |
| ����1��ȷ��ȡһ�������ĵ������岢����������ˮ�ܽ⣮ ����2����������Һת�Ƶ�����X�У�������ˮ��ϴ�ձ��Ͳ�����2��3�Σ���ϴ��ҺҲת�Ƶ�X�У� ����3��������X�м�����ˮ��Һ�� ��X�Ŀ̶���l��2cm���� |
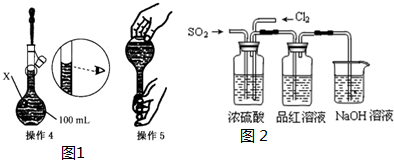
������X��������100mL����ƿ��
�ڹ��ڸ����ƹ��̣�����˵����ȷ����AB��
A������1�У�Ӧ��ȡ�������������Ϊ2.5g
B������2�У�ϴ��Һ��Ҫת�Ƶ�����X��
C������4��Ϊ���ݣ����ڸ�ͬѧ�۲췽������ȷ��������������ҺŨ��ƫ��
D������5ҡ�Ⱥ��ã�����Һ����ڿ̶��ߣ�Ӧ������ˮ����Һ����̶�������
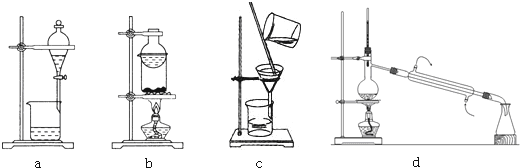
��2���ס���ͬѧ����ͼװ����֤SO2��Cl2��Ư���ԣ���ͬѧ��1��1ͨ��ʱ��Ʒ����Һ��������ɫ��Ʒ����Һ����ɫ��ԭ���ǣ��÷���ʽ��ʾ����SO2+Cl2+2H2O=SO42-+2Cl-+4H+������ͬѧ��ʵ��������Ʒ����Һ��ʱ������Ʊ��Խ��Խdz����ͬѧ������Ʒ����Һ���Խ��Խdz��ԭ�������Ӷ��������ͨ��������������ͨ������
| A�� | NaOH��Һ | B�� | Na2CO3��Һ | C�� | NaHSO3��Һ | D�� | KI��Һ |
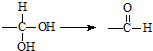 �����
���л���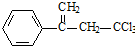 ��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ죮�����йظ��л����˵������ȷ���ǣ�������
��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ죮�����йظ��л����˵������ȷ���ǣ�������| A�� | ����±��������ʹ���Ը��������Һ����ˮ��ɫ | |
| B�� | �����ʼ��ж�ӳ�칹��Ҳ��˳���칹 | |
| C�� | �ڼ��������³��ˮ�⣬������������ | |
| D�� | 1mol ��������һ�������¿���4molH2�����ӳɷ�Ӧ |

 ��
�� 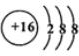 ��D��E���γ�һ������ԭ������������8���ӵķ��ӣ��÷��ӵĽṹʽΪS=C=S��D������Ԫ�ص��⻯���У��е���͵���H2S��
��D��E���γ�һ������ԭ������������8���ӵķ��ӣ��÷��ӵĽṹʽΪS=C=S��D������Ԫ�ص��⻯���У��е���͵���H2S��