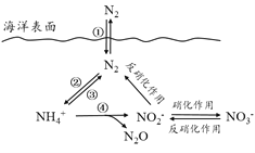
ĖâÄŋÄÚČÝ
ĄūĖâÄŋĄŋÏÂąíĘĮÔŠËØÖÜÆÚąíĩÄŌŧēŋ·ÖĢŽĮëÓÃŧŊŅ§ÓÃÓïŧØīðÓÐđØÎĘĖâĢš
ĒņA | ĒōA | ĒóA | ĒôA | ĒõA | ĒöA | ĒũA | 0 | |
1 | ĒŲ | |||||||
2 | ĒÚ | ĒÛ | ĒÜ | ĒÝ | ||||
3 | ĒÞ | Ēß | Ēā | Ēá |
ĢĻ1ĢĐĒÞĩÄĮâŅõŧŊÎïÖÐĢŽËųšŽŧŊŅ§žüĩÄĀāÐÍĘĮ______ĄĢ
ĢĻ2ĢĐĒŲšÍĒÜŋÉÐÎģÉŧŊšÏÎïĢŽÓÃĩįŨÓĘ―ąíĘūÆäÐÎģÉđýģĖ______ĄĢ
ĢĻ3ĢĐĒßĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎïÓëĒāĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎï·īÓĶĩÄĀëŨÓ·―ģĖĘ―ÎŠ_____ĄĢ
ĢĻ4ĢĐŅÐūŋÎïÖĘĩÄÐÔÖĘēîŌėÐÔĘĮŅ§Ï°ĩÄÖØŌŠ·―·ĻÖŪŌŧĄĢĒÚĄĒĒÛĄĒĒāĄĒĒáËÄÖÖÔŠËØĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎïÖÐĢŽŧŊŅ§ÐÔÖĘÃũÏÔēŧÍŽÓÚÆäËüČýÖÖËáĩÄĘĮ______ĄĢ
ĢĻ5ĢĐÄÜËĩÃũÔŠËØĒÝĩÄ·Į―ðĘôÐÔĮŋÓÚÔŠËØĒáĩÄ·Į―ðĘôÐÔĩÄĘĩŅéĘÂĘĩĘĮ_____ĢĻĖîŨÖÄļĢĐĄĢ
A.Á―ÖÖĩĨÖĘĩÄČÛ·ÐĩãēŧÍŽ
B.Á―ÖÖĩĨÖĘÓëĮâÆøŧŊšÏĩÄÄŅŌŨģĖķČ
C.ąČ―ÏÕâÁ―ÖÖÔŠËØĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎïĩÄËáÐÔ
Ąūīð°ļĄŋĀëŨÓžüĄĒđēžÛžü ![]() ŧō
ŧō![]() Al(OH)3+3H+=Al3++3H2O H2CO3 B
Al(OH)3+3H+=Al3++3H2O H2CO3 B
Ąū―âÎöĄŋ
ļųūÝļũÔŠËØÔÚÖÜÆÚąíÖÜĩÄÎŧÖÃŋÉÖŠĢŽĒŲΊHÔŠËØĢŽĒÚΊCÔŠËØĢŽĒÛΊNÔŠËØĢŽĒÜΊOÔŠËØĢŽĒÝΊFÔŠËØĢŽĒÞΊNaÔŠËØĢŽĒßΊAlÔŠËØĢŽĒāΊSÔŠËØĢŽĒáΊClÔŠËØĢŽ―ášÏÔŠËØž°ÆäŧŊšÏÎïĩÄ―áđđÓëÐÔÖĘ·ÖÎö―âīðÎĘĖâĄĢ
(1)ļųūÝÉÏĘö·ÖÎöŋÉÖŠĢŽĒÞΊNaÔŠËØĢŽÆäĮâŅõŧŊÎïΊNaOHĢŽÆäÖÐNa+šÍOH-ÐÎģÉĀëŨÓžüĢŽOH-ÖÐOÔŨÓšÍHÔŨÓÐÎģÉđēžÛžüĢŽđĘīð°ļΊĢšĀëŨÓžüĄĒđēžÛžüĢŧ
(2)HšÍOŋÉÐÎģÉĩÄŧŊšÏÎïÓÐH2OšÍH2O2ĢŽÓÃĩįŨÓĘ―ąíĘūÐÎģÉđýģĖ·ÖąðΊ![]() ĄĒ
ĄĒ![]() ĢŽđĘīð°ļΊĢš
ĢŽđĘīð°ļΊĢš![]() ŧō
ŧō![]() Ģŧ
Ģŧ
(3)AlĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎïΊAl(OH)3ĢŽSĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎïΊH2SO4ĢŽÁ―Õß·īÓĶÉúģÉAl2(SO4)3šÍH2OĢŽ·īÓĶĩÄĀëŨÓ·―ģĖĘ―ÎŠAl(OH)3+3H+=Al3++3H2OĢŽđĘīð°ļΊĢšAl(OH)3+3H+=Al3++3H2OĢŧ
(4)CĄĒNĄĒSĄĒClËÄÖÖÔŠËØĩÄŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎï·ÖąðΊĢšH2CO3ĄĒHNO3ĄĒH2SO4ĄĒHClO4ĢŽÆäÖÐH2CO3ΊČõËáĢŽķøHNO3ĄĒH2SO4ĄĒHClO4ūųΊĮŋËáĢŽđĘīð°ļΊĢšH2CO3Ģŧ
(5)ĒÝĩÄĩĨÖĘΊF2ĢŽĒáĩÄĩĨÖĘΊCl2Ģš
AĢŪĩĨÖĘĩÄČÛ·ÐĩãļúÁ―ÕßĩÄ·ķĩÂŧŠÁĶÓÐđØĢŽÓë·Į―ðĘôÐÔÎÞđØĢŽAēŧŅĄĢŧ
BĢŪ·Į―ðĘôÐÔÔ―ĮŋĢŽĩĨÖĘÓëĮâÆøŧŊšÏÔ―ČÝŌŨĢŽŌōīËŋÉÍĻđýÁ―ÖÖĩĨÖĘÓëĮâÆøŧŊšÏĩÄÄŅŌŨģĖķČËĩÃũ·Į―ðĘôĮŋČõĢŽBŅĄĢŧ
CĢŪFÃŧÓÐÕýžÛĢŽÔōēŧÄÜÍĻđýŨîļßžÛŅõŧŊÎïĩÄËŪŧŊÎïĩÄËáÐÔËĩÃũ·Į―ðĘôĮŋČõĢŽCēŧŅĄĢŧ
đĘīð°ļΊĢšBĄĢ

ĄūĖâÄŋĄŋŌŌËáķĄõĨĘĮÖØŌŠĩÄŧŊđĪÔÁÏĄĢĘĩŅéĘŌÓÃŌŌËáĄĒķĄīžÔÚÅĻÁōËáŨũīßŧŊžÁĄĒžÓČČĖõžþÏÂÖÆąļŌŌËáķĄõĨĩÄŨ°ÖÃĘūŌâÍž(žÓČȚ͞ÐģÖŨ°ÖÃŌŅĘĄÂÔ)šÍÓÐđØÐÅÏĒČįÏÂĢš
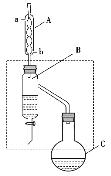
ŌŌËá | ÕýķĄīž | ŌŌËáķĄõĨ | |
ČÛĩã/Ąæ | 16.6 | -89.5 | -73.5 |
·Ðĩã/Ąæ | 117.9 | 117 | 126.0 |
ÃÜķČ/gĄĪcm-3 | 1.1 | 0.80 | 0.88 |
ÏÂÁÐËĩ·ĻÕýČ·ĩÄĘĮĢĻ ĢĐ
A.žÓČČŌŧķÎĘąžäšóĢŽ·ĒÏÖÉÕÆŋCÖÐÍüžĮžÓ·ÐĘŊĢŽŋÉīōŋŠÆŋČûÖą―ÓžÓČëžīŋÉ
B.Ũ°ÖÃBĩÄŨũÓÃĘĮēŧķÏ·ÖĀëģöŌŌËáķĄõĨĢŽĖáļßēúÂĘ
C.Ũ°ÖÃAŋÉŌÔŨ°ÅäÕôÁóŨ°ÖÃĩÄĀäÄýÆũĢŽĮŌĀäÄýËŪÓÉaŋÚ―øĢŽbŋÚģö
D.ŌŌËáķĄõĨÖÐēÐÁôĩÄŌŌËášÍÕýķĄīžŋÉÓÃąĨšÍĖžËáÄÆČÜŌšģýČĨ
ĄūĖâÄŋĄŋÄģÐĄŨéŅÐūŋĖúÓëËŪÕôÆøĩÄ·īÓĶĢŽÁ―ÎŧÍŽŅ§·Öąð―øÐÐÁËČįÏÂĘĩŅéĄĢ
ĘĩŅéĒņ | ĘĩŅéĒō |
|
|
ĮëŧØīðĢš
ĢĻ1ĢĐĘĩŅéĒņÖÐĘŠÃÞŧĻĩÄŨũÓÃĘĮ______________ĄĢ
ĢĻ2ĢĐĘĩŅéĒņÖзīÓĶĩÄŧŊŅ§·―ģĖĘ―ĘĮ__________ĄĢ
ĢĻ3ĢĐžŨÍŽŅ§đÛēėĩ―ĘĩŅéĒņÖÐģÖÐøēúÉú·ĘÔíÅÝĢŽĘĩŅéĒōÖÐČÜŌšBģĘÏÖšėÉŦĄĢËĩÃũČÜŌšAÖКŽÓÐ___________ĄĢ
ĢĻ4ĢĐŌŌÍŽŅ§đÛēėĩ―ĘĩŅéĒņÖÐģÖÐøēúÉú·ĘÔíÅÝĢŽĩŦĘĩŅéĒōÖÐČÜŌšBÎīģĘÏÖšėÉŦĄĢČÜŌšBÎīģĘÏÖšėÉŦĩÄÔŌōĘĮ____________ĄĢ