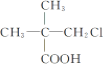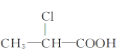题目内容
【题目】氨气是重要的化工原料。
(1)已知: N2(g)+O2(g)=2NO(g) △H= +180.5kJ·mol-1
4NH3(g)+5O2(g)=4NO(g)+6H2O(g) △H= -905kJ·mol-1
2H2(g)+O2(g)=2H2O(g) △H= -483.6kJ·mol-1
写出氨气在高温高压催化剂条件下生成氮气和氢气的热化学方程式:_____________________;如果在1 L密闭容器中,3mol NH3在等温条件下充分反应,2min后达到平衡,平衡时吸收的热量为92.4 kJ ,则在这段时间内v(H2)=___________________;保持温度不变,将起始NH3的物质的量调整为8 mol,平衡时NH3的转化率为_________________。
(2)氨气在纯氧中燃烧,生成一种单质和水,试写出该反应的化学方程式:___________________,科学家利用此原理,设计成氨气一氧气燃料电池,则通入氨气的电极是_____________(填“正极”或“负极”);碱性条件下,该电极发生反应的电极反应式为______________________________________。
(3)一定条件下,某密闭容器中发生反应:4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g) △H<0。在一定体积的密闭容器中,为使该反应的反应速率增大,且平衡向正反应方向移动,下列措施中可采用的是____________(填字母代号)。
4NO(g)+6H2O(g) △H<0。在一定体积的密闭容器中,为使该反应的反应速率增大,且平衡向正反应方向移动,下列措施中可采用的是____________(填字母代号)。
a.增大压强 b.适当升高温度 c.增大O2的浓度 d.选择高效催化剂
(4)如果某氨水的电离程度为1%,向浓度为0.01 mol/L MgCl2溶液中滴加氨水,则开始产生沉淀时(忽略溶液体积变化)溶液中的NH3·H2O的浓度为______________(已知Ksp[Mg(OH)2]=4.010-12])。
【答案】2NH3(g)![]() N2(g)+ 3H2(g) ΔH= +92.4 kJ/ mol 1.5 mol·L-1·min-1 50% 4NH3+3O2 == 2N2+6H2O 负极 2NH3+6OH--6e- = N2+6H2O C 0.002 mol/L
N2(g)+ 3H2(g) ΔH= +92.4 kJ/ mol 1.5 mol·L-1·min-1 50% 4NH3+3O2 == 2N2+6H2O 负极 2NH3+6OH--6e- = N2+6H2O C 0.002 mol/L
【解析】
(1)已知:①N2(g)+O2(g)=2NO(g)△H=+180.5kJmol-1,②4NH3(g)+5O2(g)=4NO(g)+6H2O(g)△H=-905kJmol-1,③2H2(g)+O2(g)=2H2O(g)△H=-483.6kJmol-1,根据盖斯定律,(②-①×2-③×3)÷2可得:2NH3(g)N2(g)+3H2(g),由此计算△H;
如果在1L密闭容器中,3mol NH3 在等温条件下充分反应,平衡时的反应热为92.4kJ,说明反应的氨气为2mol,则:
2NH3(g)N2(g)+3H2(g)
起始量(mol):3 0 0
变化量(mol):2 1 3
平衡量(mol):1 1 3
根据v=![]() 计算v(H2);
计算v(H2);
保持温度不变,平衡常数不变,根据K=![]() 计算平衡常数,将起始NH3的物质的量调整为8mol,设转化的氨气物质的量为xmol,表示出平衡时各组分物质的量,再结合平衡常数列方程计算解答;
计算平衡常数,将起始NH3的物质的量调整为8mol,设转化的氨气物质的量为xmol,表示出平衡时各组分物质的量,再结合平衡常数列方程计算解答;
(2)氨气在纯氧中燃烧,生成一种单质和水,应生成氮气与水;氨气--氧气燃料电池,燃料在负极发生氧化反应,则通入氨气的电极是负极,碱性条件下,生成氮气与水;
(3)a.增大压强,反应速率增大,平衡向气体体积减小的方向移动;
b.适当升高温度,反应速率增大,平衡向吸热反应移动;
c.增大反应物氧气的浓度,平衡正向进行,反应速率增大;
d.选择高效催化剂只能改变化学反应速率,但不影响化学平衡;
(4)溶液中c(Mg2+)=0.01mol/L,根据溶度积常数Ksp=c(Mg2+)×c2(OH-)=4.0×10-12 ,计算c(OH-),氨水的浓度=![]() 。
。
(1)已知:①N2(g)+O2(g)=2NO(g)△H=+180.5kJmol-1,②4NH3(g)+5O2(g)=4NO(g)+6H2O(g)△H=-905kJmol-1,③2H2(g)+O2(g)=2H2O(g)△H=-483.6kJmol-1,依据盖斯定律,(②-①×2-③×3)÷2可得:2NH3(g)N2(g)+3H2(g),△H=+92.4 kJmol-1;
如果在1L密闭容器中,3mol NH3 在等温条件下充分反应,平衡时的反应热为92.4kJ,说明反应的氨气为2mol,则:
2NH3(g)N2(g)+3H2(g)
起始量(mol):3 0 0
变化量(mol):2 1 3
平衡量(mol):1 1 3
v(H2)=![]() =1.5mol/(Lmin);
=1.5mol/(Lmin);
平衡常数K=![]() =
=![]() =27,保持温度不变,平衡常数不变,将起始NH3的物质的量调整为8mol,设转化的氨气物质的量为xmol,则:
=27,保持温度不变,平衡常数不变,将起始NH3的物质的量调整为8mol,设转化的氨气物质的量为xmol,则:
2NH3(g)N2(g)+3H2(g)
起始量(mol):8 0 0
变化量(mol):x 0.5x 1.5x
平衡量(mol):8-x 0.5x 1.5x
则![]() =27,解得x=4 ,平衡时氨气的转化率=
=27,解得x=4 ,平衡时氨气的转化率=![]() ×100%=50%;
×100%=50%;
(2)氨气在纯氧中燃烧,生成一种单质和水,应生成氮气与水,反应的化学方程式:4NH3+3O2 ![]() 2N2+6H2O;氨气--氧气燃料电池,燃料在负极发生氧化反应,则通入氨气的电极是负极,碱性条件下生成氮气与水,该电极发生反应的电极反应式为:2NH3+6OH--6e-=N2+6H2O;
2N2+6H2O;氨气--氧气燃料电池,燃料在负极发生氧化反应,则通入氨气的电极是负极,碱性条件下生成氮气与水,该电极发生反应的电极反应式为:2NH3+6OH--6e-=N2+6H2O;
(3)a.反应是气体体积增大的反应,增大压强,反应速率增大,平衡逆向进行,故a不符合;b.反应是放热反应,适当升高温度,反应速率增大,平衡逆向进行,故b不符合;c.增大O2的浓度,平衡正向进行,反应速率增大,故c符合;d.选择高效催化剂只能改变化学反应速率,但不改变化学平衡,故d不符合;故答案为c;
(4)如果某氨水的电离程度为1%,浓度为0.01mol/LMgCl2溶液滴加氨水至开始产生沉淀时(不考虑溶液体积变化),{已知Ksp[Mg(OH)2]=4.0×10-12]},则依据:溶度积常数Ksp=c(Mg2+)×c2(OH-)=4.0×10-12 ,溶液中c(Mg2+)=0.01mol/L,则c(OH-)=2×10-5mol/L,氨水的浓度=![]() =0.002mol/L。
=0.002mol/L。

 阅读快车系列答案
阅读快车系列答案【题目】一定温度时,向容积为2L的密闭容器中充入一定量的SO2和O2,发生反应2SO2(g)+O2(g)![]() 2SO3(g) H=-196kJ·moL1,一段时间后达平衡,反应过程中测定的部分数据见下表:
2SO3(g) H=-196kJ·moL1,一段时间后达平衡,反应过程中测定的部分数据见下表:
反应时间/min | n(SO2)/mol | n(O2)/mol |
0 | 2 | 1 |
5 | 1.2 | |
10 | 0.4 | |
15 | 0.8 |
下列说法不正确的是
A. 反应在前5min的平均速率为v (SO2)=0.08mol·L1·min1
B. 保持温度不变,向平衡后的容器中再充入1molSO2和0.5molO2时,v (正)> v (逆)
C. 该温度,反应的平衡常数为11.25L·mol-1
D. 相同温度下,起始时向容器中充入1.5mol SO3,达平衡时SO3的转化率为40%