题目内容
【题目】HIO3是强酸,其水溶液是强氧化剂。工业上,以KIO3为原料可制备HIO3。某学习小组拟用如图装置制备碘酸。M、N为惰性电极,ab、cd为交换膜。下列推断错误的是( )
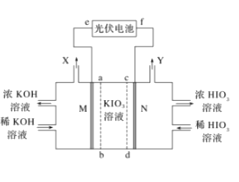
A.光伏电池的e极为负极,M极发生还原反应
B.在标准状况下收集6720mLX和Y的混合气体时KIO3溶液减少3.6g
C.Y极的电极反应式为2H2O-4e-═O2↑+4H+
D.制备过程中要控制电压,避免生成HIO4等杂质
【答案】B
【解析】
根据图示,左室增加KOH,右室增加HIO3,则M室为阴极室,阴极与外加电源的负极相接,电极反应式为2H2O+2e-=H2↑+2OH-,所以原料室中K+透过ab膜进入阴极室生成KOH,即ab膜为阳离子交换膜,N室为阳极室,原料室中IO3-透过cd膜进入阳极室生成HIO3,即cd膜为阴离子交换膜。
A.由上述分析可知,M室为阴极室,阴极与外加电源的负极相接,即e极为光伏电池负极,阴极发生得到电子的还原反应,故A正确;
B.N室为阳极室,与外加电源的正极相接,电极反应式为2H2O-4e-=O2↑+4H+,M极电极反应式为2H2O+2e-=H2↑+2OH-,标准状况下6720mL气体即6.72L气体物质的量为6.72L÷22.4L/mol=0.3mol,其中含有O2为0.1mol,转移电子0.4mol,为平衡电荷,KIO3溶液中0.4molK+透过ab膜进入阴极室,0.4molIO3-透过cd膜进入阳极室,KIO3溶液质量减少0.4mol×214g/mol=85.6g,故B错误;
C.N室为阳极室,与外加电源的正极相接,电极反应式为2H2O-4e-=O2↑+4H+,故C正确;
D.制备过程中若电压过高,阳极区(N极)可能发生副反应:IO3--2e-+H2O=IO4-+2H+,导致制备的HIO3不纯,所以制备过程中要控制电压适当,避免生成HIO4等杂质,故D正确;
故答案选B。

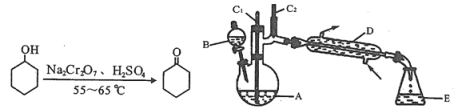
【题目】实验室可利用环己醇的氧化反应制备环己酮,反应原理和实验装置(部分夹持装置略)如下:
有关物质的物理性质见下表。
物质 | 沸点(℃) | 密度(g·cm-3,20℃) | 溶解性 |
环己醇 | 161.1(97.8)* | 0.96 | 能溶于水和醚 |
环己酮 | 155.6(95.0)* | 0.95 | 微溶于水,能溶于醚 |
水 | 100.0 | 1.0 |
*括号中的数据表示该有机物与水形成的具有固定组成的混合物的沸点。
实验中通过装置B将酸性Na2Cr2O7溶液加到盛有10 mL环己醇的A中,在55 ~ 60℃进行反应。反应完成后,加入适量水,蒸馏,收集95 ~ 100℃的馏分,得到主要含环己酮粗品和水的混合物。
(1)装置D的名称为____________________________。
(2)酸性Na2Cr2O7溶液氧化环己醇反应的![]() ,反应剧烈将导致体系温度迅速上升,副反应增多。
,反应剧烈将导致体系温度迅速上升,副反应增多。
①滴加酸性Na2Cr2O7溶液的操作为____________________________________________;
②蒸馏不能分离环己酮和水的原因是__________________________________________。
(3)环己酮的提纯需要经过以下一系列的操作:
a.蒸馏、除去乙醚后,收集151~156℃馏分
b.水层用乙醚(乙醚沸点34.6℃,易燃烧)萃取,萃取液并入有机层
c.过滤
d.往液体中加入NaCl固体至饱和,静置,分液
e.加人无水MgSO4固体,除去有机物中少量的水
①上述提纯步骤的正确顺序是________________________________________________;
②B中水层用乙醚萃取的目的是______________________________________________;
③上述操作c、d中使用的玻璃仪器除烧杯、锥形瓶、玻璃棒外,还需要的玻璃仪器有__________,操作d中,加入NaC1固体的作用是_____________________________。
(4)恢复至室温时,分离得到纯产品体积为6 mL,则环已酮的产率为____________。(计算结果精确到0.1%)