��Ŀ����
12��С��ͬѧ��ʵ���ҷ���һƿ���ڷ��õ�NaOH���壬������ƿNaOH����ı����������������ʵ��̽������1��ȡ�����������Թ��У��μ�ϡ���ᣬ�����ݲ������ɴ˿�֪��NaOH�����ѱ��ʣ�д�����������ڿ����б��ʵĻ�ѧ����ʽ2NaOH+CO2=Na2CO3+H2O
��2����ͬѧΪ��һ��̽���������Ƿ���NaOH�����������ʵ�鷽����
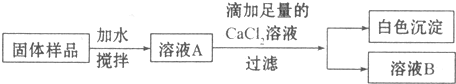
�ٵμ�������CaCl2��ҺĿ���dz�����Һ�е�̼���ƣ���CO32-�������ɳ����Ļ�ѧ����ʽΪCaCl2+Na2CO3=CaCO3��+2NaCl��
��ȡ������ҺB���Թ��У��μ�������̪��Һ������Ϊ��죨��ɫ����������ǹ�����Ʒ�д��ڣ����ڣ� ������ڡ������ڡ����������ƣ�
���� ��1��NaOH�������տ����еĶ�����̼�����ʣ�
��2���ٸ����������Ƴ��ڷ��ú���������̼���ƣ��ݴ˷�������������CaCl2��Һ��Ŀ�ĺͷ�Ӧ��
��̽���������Ƿ���NaOH����Ϊ̼������ҺҲ�Լ��ԣ���Ҫ���ų�̼���Ƶĸ��ţ�Ȼ�������Һ�мӷ�̪����ɫ�仯���ж��������ƵĴ��ڣ�
��� �⣺��1��NaOH�����ڿ����г��ڷ��ã��������տ����еĶ�����̼�����ʣ�2NaOH+CO2=Na2CO3+H2O���ʴ�Ϊ��2NaOH+CO2=Na2CO3+H2O��
��2����������CaCl2��Һ�ɽ�̼����ȫ����Ӧ�����μ�������CaCl2��ҺĿ���ǣ�������Һ�е�̼���ƣ���CO32-�������ɳ����Ļ�ѧ����ʽΪCaCl2+Na2CO3=CaCO3��+2NaCl���ʴ�Ϊ��������Һ�е�̼���ƣ���CO32-����CaCl2+Na2CO3=CaCO3��+2NaCl��
�����̪������ɫ���������������ƣ��ӷ�̪��Һ��죬��֮������죻�ʴ�Ϊ����̪����죨��ɫ�������ڣ����ڣ���
���� ͨ������ѧ��Ҫ��ȷ�����ɷ֣���Ҫ�Ƿ������ܷ����Ļ�ѧ��Ӧ��Ӧ��Ϥ����֮������Ӧ������

��ϰ��ϵ�д�
�����Ŀ
2����֪��CO��g��+H2O��g���TCO2��g��+H2��g����H=-41kJ/mol����ͬ�¶��£��������Ϊ2L�����������ܱ������У�����һ�����ķ�Ӧ�����Ӧ������������£�
����˵���У�����ȷ���ǣ�������
| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ�������ϵ�����ı仯 | |||
| n��CO�� | n��H2O�� | n��CO2�� | n��H2�� | ||
| �� | 1 | 4 | 0 | 0 | �ų�������32.8 kJ |
| �� | 0 | 0 | 1 | 4 | �����仯��Q1 |
| �� | 1 | 1 | 2 | 1 | �����仯��Q2 |
| A�� | ���������з�Ӧ10min�ﵽƽ�⣬0��10minʱ���ڣ���CO��ʾ��ƽ����Ӧ���ʦԣ�CO��=4.0��10-2 mol/��L•min�� | |
| B�� | �������У���ʼʱ�ԣ�CO���������ԣ�CO������ | |
| C�� | ��ƽ�������ϵ�����ı仯��Q1=4Q2 | |
| D�� | ƽ��ʱ�������������CO������������ |
3���������ķ�չ���ǵ�����Խ��Խ�벻����ѧ������˵����ȷ���ǣ�������
| A�� | ���·������������ۻ�Ϊͬ���칹�� | |
| B�� | �Ʒ��ס����С���ѹ���ȵIJ�����ǺϽ� | |
| C�� | ��ըʳ��Ļ����ͺ�ţ�Ͷ��ǿ������ı������� | |
| D�� | ĥ�����Ĵ��������ʣ�������к��ʱ���˰����� |
20�����м�ͥСʵ���ܳɹ����ǣ�������
| A�� | �ô������ķ��¸����Ƶ��㹳 | B�� | ��ʳ��ˮ��ȥ��ˮƿ�е�ˮ�� | ||
| C�� | �õ�ء�пƤ����ʳ��Ӧ������ | D�� | �ü��ȵķ�������ľ���� |
4�����ڿ��淴Ӧ��2A��g��+2B��g��?3C��g��+D��s��������ѹǿ������Ӱ����ǣ�������
| A�� | ����Ӧ���ʼӿ죬�淴Ӧ���ʼ�����ƽ��������Ӧ�����ƶ� | |
| B�� | �淴Ӧ���ʼӿ죬����Ӧ���ʼ�����ƽ�����淴Ӧ�����ƶ� | |
| C�� | �����淴Ӧ���ʶ����䣬ƽ�����淴Ӧ�����ƶ� | |
| D�� | �����淴Ӧ���ʶ�������ƽ�����淴Ӧ�����ƶ� |
1�������й�˵����ȷ���ǣ�������
| A�� | Ư�۾����ڿ����л�����Ư��Ч�� | |
| B�� | �Ժ�ˮΪԭ������ȡNa��HCl��Mg��Br2������ | |
| C�� | ��ͭ��[Cu3��OH��2��CO3��2]Ҳ�ɱ�ʾΪ3CuO•2CO2•2H2O | |
| D�� | �������費���κ��ᷴӦ������ʯӢ������������ |
2���������ӷ���ʽ��д��ȷ���ǣ�������
| A�� | ��ƫ��������Һ�еμӹ������AlO2-+H++H2O=A1��OH��3�� | |
| B�� | ̼��������Һ��������ʯ��ˮ��Ca2++2HCO3-+2OH-=CaCO3��+CO32-+2H2O | |
| C�� | �����Ȼ�����Һ�м������ͭм��2Fe3++3Cu=2Fe+3Cu2+ | |
| D�� | ��NaOH��Һ��������Cl2���壺OH-+Cl2=Cl-+HClO |

 �������ı�ʾ����ΪO2-��
�������ı�ʾ����ΪO2-��