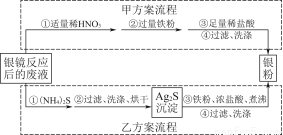
جâؤ؟ؤعبف
¶آب»¯¶ءٍ£¨S2Cl2£©تا¹م·؛سأسعدً½؛¹¤زµµؤءٍ»¯¼ء£¬ئن·ض×س½ل¹¹بçح¼ثùت¾،£³£خآدآ£¬S2Cl2تاز»ضض³ب»ئة«ز؛جه£¬سِث®ز×ث®½â£¬²¢²ْةْؤـت¹ئ·؛ىحتة«µؤئّجه£¬ »¯ر§·½³جت½خھ2S2Cl2£«2H2O=SO2،ü£«3S،£«4HCl،£دآءذثµ·¨ضذ´يخَµؤتا£¨،،،،£©

A£®S2Cl2µؤ½ل¹¹ت½خھCl،ھS،ھS،ھCl
B£®·´س¦ضذSO2تا»¹ش²ْخSتارُ»¯²ْخï
C£®S2Cl2خھ؛¬سذ¼«ذش¼ü؛ح·ا¼«ذش¼üµؤ·ض×س
D£®Sش×س²ةب،sp3شس»¯
B
،¾½âخِ،؟·´س¦ضذءٍشھثطµؤ»¯؛د¼غسة£«1±ن³ة£«4¼غ؛ح0¼غ£¬·¢ةْµؤتاءٍشھثطµؤئ绯·´س¦£¬SO2تارُ»¯²ْخSتا»¹ش²ْخBدî´يخَ£»ءٍ،ھءٍ¼üخھ·ا¼«ذش¼ü£¬ءٍ،ھآب¼üخھ¼«ذش¼ü£¬·ض×س²»¶ش³ئ£¬خھ¼«ذش·ض×س£¬Cدîصب·£»ءٍش×سµؤ¼غµç×سخھ6¸ِ£¬ذخ³ةءثء½¸ِ¦ز¼ü£¬»¹سذ4¸ِµç×سخ´²خسë³ة¼ü£¬خھء½¸ِ¹آµç×س¶ش£¬¹ت²ةب،sp3شس»¯£¬Dدîصب·،£

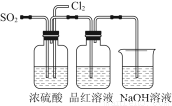
خھءثج½¾؟SO2سëNa2O2µؤ·´س¦تا·ٌہàثئسعCO2سëNa2O2µؤ·´س¦£¬¼×ح¬ر§ةè¼ئءثبçح¼ثùت¾µؤتµرé×°ضأ£¬»ط´ًدآءذختجâ£؛

£¨1£©زئ؟ھأق»¨£¬½«´ّ»ًذاµؤؤ¾جُ·إشعCتش¹ـ؟ع£¬خ´¼ûؤ¾جُ¸´ب¼£¬¼×ح¬ر§زٍ´ثبدخھSO2سëNa2O2µؤ·´س¦²»ح¬سعCO2،£اë°´¼×ح¬ر§µؤ¹غµمذ´³ِ·´س¦µؤ»¯ر§·½³جت½ ،£
£¨2£©ززح¬ر§بدخھخقآغ·´س¦شہيبç؛خ£¬×îضص¶¼سذO2²ْةْ£¬ززح¬ر§µؤہيسةتا ،£°´صصززح¬ر§µؤ¹غµم£¬¸أ×°ضأذè×ِµؤ¸ؤ½ّتا
،£
£¨3£©¼ظةèNa2O2حêب«·´س¦£¬·´س¦؛َB×°ضأضذ¹ججهةْ³ةخï؟ةؤـتا£؛¢ظNa2SO3£»¢عNa2SO4£»¢غNa2SO3؛حNa2SO4،£
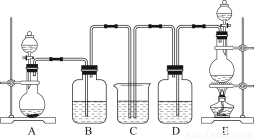
اëةè¼ئتµرé·½°¸¼ىر飬ذ´³ِتµرé²½ضèزش¼°ش¤ئعدضدَ؛ح½لآغ£¬حê³ةدآ±ي£؛
دقر،تش¼ء£؛2 mol،¤L£1 HClبـز؛£¬1 mol،¤L£1 HNO3بـز؛£¬1 mol،¤L£1 BaClبـز؛£¬1 mol،¤L£1 Ba£¨NO3£©2بـز؛£¬0.01 mol،¤L£1 KMnO4ثلذشبـز؛،£
تµرé²½ضè | ش¤ئعدضدَ؛ح½لآغ |
²½ضè1£؛ب،Bضذµؤةظء؟¹ججهرùئ·سعتش¹ـضذ£¬µخ¼س×مء؟صôءَث®£¬بـ½â£¬ب»؛َب،ةظء؟´²âز؛·ض±ًضأسع¢ٌ،¢¢ٍتش¹ـضذ | ¹ججهحêب«بـ½â |
²½ضè2£؛حù¢ٌتش¹ـضذ¼سبë £¬شظµخ¼س | £¬ |
شٍض¤أ÷ةْ³ةخïضذ؛¬Na2SO4 |
|
²½ضè3£؛حù¢ٍتش¹ـضذ |
|
| بô £¬ |
شٍض¤أ÷ةْ³ةخïضذسذNa2SO3£»بô |
|
|
|
شٍثµأ÷ةْ³ةخïضذأ»سذNa2SO3،£ |
|
£¨4£©ةْ³ةخïضذراءٍثلؤئ؛¬ء؟µؤ²â¶¨£؛
¢ظب،a gةْ³ةخïإنضئ³ة100 mLبـز؛£¬ب،10.00 mL¸أبـز؛سع׶ذخئ؟ضذ£¬¼سب뼸µخµي·غبـز؛×÷ض¸ت¾¼ء£¬سأ0.010 0 mol،¤L£1µâث®½ّذذµخ¶¨£¬µخ¶¨ضصµمدضدَخھ ،£¼اآ¼ت¾ف£¬ضط¸´µخ¶¨2´خ£¬ئ½¾ùدû؛ؤµâث®20.00 mL،£
¢ع¼ئثم£؛ةْ³ةخïضذراءٍثلؤئµؤضتء؟·ضتخھ ،£