��Ŀ����
4�� �ǽ���Ԫ��H��C��N��O��S��Cl���γɵĻ���������ܶ࣬���ʼ����������;�ܹ㷺��������и��⣮
�ǽ���Ԫ��H��C��N��O��S��Cl���γɵĻ���������ܶ࣬���ʼ����������;�ܹ㷺��������и��⣮��1����O2-�ĺ�������Ų�ʽΪ1s22s22p6��CS2�ľ�������Ϊ���� ���壬��C�Ĺ���ӻ���ʽΪsp
��CH3OH�ڳ�����ΪҺ̬���е�����������Ҫԭ���Ǽ״����Ӽ����γ������������Ӽ䲻���γ������
��2��Cl2��һ�ִ�����Ⱦ�Һ�ȴ��������е�˵�������£����֣���
| ���� |  |
| ����Ҫ�� | Զ�������ĩ���������ࡢ�������ʣ�������������� |
| й©���� | NaOH��NaHSO3��Һ���� |
| ��װ | ��ƿ |
����Һ��й©�����������ڸ�ƿ�������뱽�ķ�Ӧ���Լӿ죬ԭ��������������Ӧ���ɵ��Ȼ����������뱽�ķ�Ӧ�д����ã�
�۽�Cl2ͨ������KOH��Һ�У������п�����KCl��KClO��KClO3������Һ��c��Cl-����c��ClO-��=11��1ʱ����c��ClO-����c��ClO3-����ֵ����$\frac{1}{2}$
��3������ʱ����100mL 0.1mol•L-1 NH4HSO4��Һ�еμ�0.1mol•L-1 NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������a����b�㣬��Һ�и�����Ũ���ɴ�С������˳����c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ��1����O2-�ĺ�����10�����ӣ����ݹ���ԭ����д�����Ӻ�������Ų�ʽ��CS2�Ĺ������Ƿ��ӣ�����̼��Cԭ�Ӽ۲���ӶԸ�����2�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж�C�Ĺ���ӻ���ʽ��
�����γɷ��Ӽ�����������۷е�ϸߣ�
��2������������ǿ�����ԣ���������ԭ�Ե�������������ӣ����������ӡ���������ӣ�
������������Ӧ�����Ȼ������Ȼ������������ӿ챽�������ķ�Ӧ��
�۸���ת�Ƶ�����ȼ�������c��ClO3-����
��3��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��b��c��d������Һ������NH3•H2O����NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3•H2O����ˮ�ĵ��룮b����Һ�����ԣ�
��� �⣺��1����O2-�ĺ�����10�����ӣ����ݹ���ԭ����д�����Ӻ�������Ų�ʽΪ1s22s22p6��CS2�Ĺ������Ƿ��ӣ����Զ���̼�Ƿ��Ӿ��壻����̼��Cԭ�Ӽ۲���ӶԸ�����2�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ�������֪C�Ĺ���ӻ���ʽΪsp��
�ʴ�Ϊ��1s22s22p6�����ӣ�sp��
�����γɷ��Ӽ�����������۷е�ϸߣ��״����Ӽ����γ������������Ӽ䲻���γ���������Լ״����۷е�������飬�ʴ�Ϊ���״����Ӽ����γ������������Ӽ䲻���γ������
��2����NaHSO3��Һ��й¶����������������ԭ��Ӧ�����ӷ�ӦΪHSO3-+Cl2+H2O�TSO42-+2Cl-+3H+���ʴ�Ϊ��HSO3-+Cl2+H2O�TSO42-+2Cl-+3H+��
�����ڸ�ƿ�������뱽�ķ�Ӧ���Լӿ��֪������������Ӧ���ɵ��Ȼ����������뱽�ķ�Ӧ�д����ã�
�ʴ�Ϊ������������Ӧ���ɵ��Ȼ����������뱽�ķ�Ӧ�д����ã�
����n��ClO-��=1mol����Ӧ��c��Cl-����c��ClO-��=11��1����n��Cl-��=11mol������ת�Ƶ�����ȵ�11mol=1mol+n��ClO3-����5��n��ClO3-��=2������c��ClO-����c��ClO3-��=1mol��2mol=$\frac{1}{2}$
�ʴ�Ϊ��$\frac{1}{2}$��
��3��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��b��c��d������Һ������NH3•H2O����NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3•H2O����ˮ�ĵ��룻b����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�a��ʱc��Na+��=c��SO42-����b��ʱc��Na+����c��SO42-��������NԪ����SԪ�صĹ�ϵ�����Եó�c��SO42-����c��NH4+������c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
�ʴ�Ϊ��a��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ���⿼������ʽṹ�����ʡ���ѧ��Ӧԭ�����漰�������Һ�����жϡ�������ԭ��Ӧ��ԭ�Ӻ�������Ų������������жϡ�ԭ���ӻ���ʽ�жϡ������֪ʶ�㣬Ϊ��Ƶ���㣬�ۺ��Խ�ǿ����������ˮ��ԭ����������ԭ��Ӧת�Ƶ����غ㡢����ԭ�����۲���ӶԻ������۵�֪ʶ�����ѵ��ǣ�3��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | һ�����м��� | B�� | ��������ϩ | ||
| C�� | һ������������ | D�� | һ��������ϩ����һ�����м��� |
| A�� | 28g N2���еĵ�ԭ����ĿΪNA | |
| B�� | ���³�ѹ�£�22.4L Cl2���еķ�����ĿΪNA | |
| C�� | 1molNa��ΪNa+ʱʧȥ�ĵ�����ĿΪNA | |
| D�� | 1L 1mol•L-1K2CO3��Һ�к��еļ�������ĿΪNA |
CO��g��+$\frac{1}{2}$O2��g��=CO2��g����H=-280.0kJ/mol��
ijH2��CO�Ļ��������ȫȼ�շų�141kJ������ͬʱ���ı�״����5.6L O2����ԭ���������H2��CO�����ʵ���֮��Ϊ��������
| A�� | 2��1 | B�� | 1��2 | C�� | 1��1 | D�� | 2��3 |
| A�� | ��ˮ����������Һ��ϣ�NH3•H2O+H+�TNH4++H2O | |
| B�� | ������SO2ͨ��Ca��ClO��2��Һ�У�SO2+H2O+Ca2++2ClO-=2HClO+CaSO3�� | |
| C�� | ������м����ϡ���2Fe+6H+=2Fe3++3H2�� | |
| D�� | ��Cl2ͨ��FeSO4��Һ�У�Cl2+2Fe2+�T2Fe3++2 Cl- |
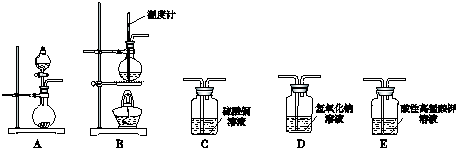
��������
| ��� | ���� | װ������˳������ĸ�� | �Ʊ���Ӧ�Ļ�ѧ����ʽ |
| ��1�� | ��ϩ | B��D��E | CH3-CH2-OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O |
| ��2�� | ��Ȳ | A��C��E | CaC2+2H2O��CH��CH��+Ca��OH��2 |
��ҵ������ϩ������Ϊԭ�ϣ������и����ϳɾ�����ϩ��PVC����
��ϩ$��_{��1��}^{һ������}$��$��_{��2��}^{һ������}$��$��_{��3��}^{һ������}$PVC
�Ľṹ��ʽ��CH2ClCH2Cl����Ӧ��3���Ļ�ѧ����ʽ��nH2C=CHCl$\stackrel{����}{��}$
 ��
�� | A�� | ������ʹ����KMnO4��Һ��ɫ��������������ƣ���˱�Ϊ������ | |
| B�� | ���Ľṹ��ʽΪ ��������˫����������ˮ�����ӳɷ�Ӧ ��������˫����������ˮ�����ӳɷ�Ӧ | |
| C�� | ����6��̼ԭ�Ӻ�6����ԭ����ͬһƽ���� | |
| D�� | ��1mL����1mLˮ��ֻ�Ϻ��ã������� |
 ��
�� ͼΪһ��ԭ��أ�����������⣺
ͼΪһ��ԭ��أ�����������⣺