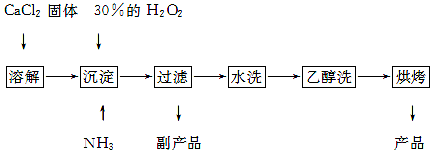
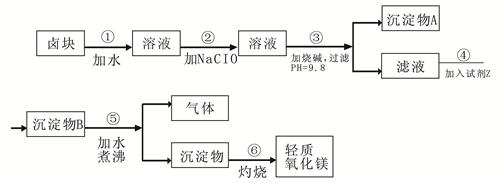
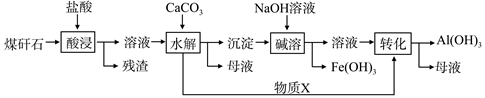
��Ŀ����
ij�о�С��������¹���·���ᴿ��ҵ̼���ơ���֪��ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl������SO42�������ʡ�

��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��
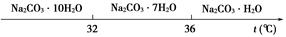
��.�й����ʵ��ܶȻ����£�
����������Ϣ���ش��������⣺
(1)����NaOH��Һʱ���������ӷ���ʽΪ___________________________��
����Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8ʱ��c(Mg2��)��c(Fe3��)��__________________________________________
(2)�����ȹ��ˡ�ʱ���¶�Ӧ������____________________________________
(3)���˴ӡ���ɫ��ѧ���ĽǶ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�á��������ʵ�ʹ�ҵ�������Ƿ����__________________����˵������_______________________________________

��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��
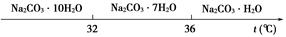
��.�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
����������Ϣ���ش��������⣺
(1)����NaOH��Һʱ���������ӷ���ʽΪ___________________________��
����Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8ʱ��c(Mg2��)��c(Fe3��)��__________________________________________
(2)�����ȹ��ˡ�ʱ���¶�Ӧ������____________________________________
(3)���˴ӡ���ɫ��ѧ���ĽǶ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�á��������ʵ�ʹ�ҵ�������Ƿ����__________________����˵������_______________________________________
(1)Fe3����3OH��=Fe(OH)3����MgCO3��2OH��=Mg(OH)2��CO32����Mg2����2OH��=Mg(OH)2��(���������������)��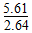 ��1021��2.125��1021
��1021��2.125��1021
(2)����36 ��
(3)�����С�����ĸҺ��ѭ��ʹ�ã�����Һc(Cl��)��c(SO42��)����������ò���Na2CO3�л�������
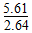 ��1021��2.125��1021
��1021��2.125��1021(2)����36 ��
(3)�����С�����ĸҺ��ѭ��ʹ�ã�����Һc(Cl��)��c(SO42��)����������ò���Na2CO3�л�������
(1)��ҵ̼�����к��е�Mg2����Fe3����������OH����Ӧ������ҺpH��8ʱ��
c(OH��)��10��6 mol��L��1��
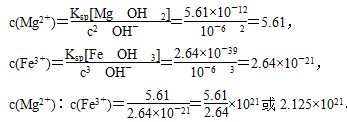
(2)�����ȹ��ˡ��õ���������Na2CO3��H2O���¶�Ӧ�����ڸ���36 �档
(3)��ĸҺ���к���Cl����SO42������ѭ��ʹ�ã�����Һc(Cl��)��c(SO42��)����������ò���Na2CO3�������ʡ�
c(OH��)��10��6 mol��L��1��
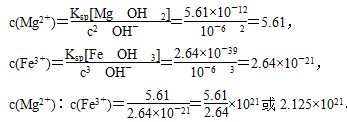
(2)�����ȹ��ˡ��õ���������Na2CO3��H2O���¶�Ӧ�����ڸ���36 �档
(3)��ĸҺ���к���Cl����SO42������ѭ��ʹ�ã�����Һc(Cl��)��c(SO42��)����������ò���Na2CO3�������ʡ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ