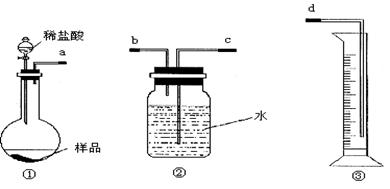
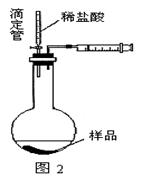
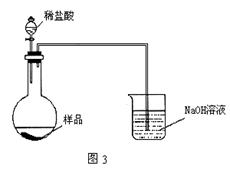
��Ŀ����
����8�֣�ij��ɫ��Һ�п��ܺ���Na����SO42�� ��MnO4����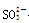 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
����pH��ֽ���飬��ҺpH��7��
������Һ�еμ���ˮ��������������ټ���CCl4������,CCl4��ʳȺ�ɫ���÷�Һ©����Һ��
�����Һ���ˮ��Һ�м���AgNO3��HNO3���Һ���а�ɫ����������
����ȡԭ��Һ��������Ba(NO3)2������Ļ��Һ��������ɫ������
�ش��������⣺
��1����Һ�п϶����е������� ���϶�û�е������� ��
��д��������е����ӷ���ʽ ��
����6�֣�SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
�� �� ��
�������ʵ��֤���� ��
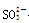 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�����pH��ֽ���飬��ҺpH��7��
������Һ�еμ���ˮ��������������ټ���CCl4������,CCl4��ʳȺ�ɫ���÷�Һ©����Һ��
�����Һ���ˮ��Һ�м���AgNO3��HNO3���Һ���а�ɫ����������
����ȡԭ��Һ��������Ba(NO3)2������Ļ��Һ��������ɫ������
�ش��������⣺
��1����Һ�п϶����е������� ���϶�û�е������� ��
��д��������е����ӷ���ʽ ��
����6�֣�SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
�� �� ��
�������ʵ��֤���� ��
����.��8�֣���Na+ ��Br����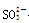
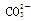 ��MnO4��
��MnO4��
��Cl2+2Br��== Br2+2 Cl��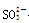 +Cl2+H2O ==2H++2Cl��+SO42��
+Cl2+H2O ==2H++2Cl��+SO42��
����.(6�֣�
�ټ���һ��SO2��SO2��ˮ���õIJ���Ư���˺�ɫ����Һ��
�ڼ������SO2��NaOH��Ӧ��������Һ��PH��������NaOH�����Լ������ɣ�
����һ��ȡ������ɫ�����Һ���Թܣ�����NaOH��Һ����Һ�ָ���ɫ��������������
��������ȡ������ɫ�����Һ���Թܣ��þƾ��Ƽ��ȣ���Һ�ָ���ɫ���������һ����
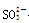
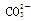 ��MnO4��
��MnO4�� ��Cl2+2Br��== Br2+2 Cl��
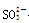 +Cl2+H2O ==2H++2Cl��+SO42��
+Cl2+H2O ==2H++2Cl��+SO42������.(6�֣�
�ټ���һ��SO2��SO2��ˮ���õIJ���Ư���˺�ɫ����Һ��
�ڼ������SO2��NaOH��Ӧ��������Һ��PH��������NaOH�����Լ������ɣ�
����һ��ȡ������ɫ�����Һ���Թܣ�����NaOH��Һ����Һ�ָ���ɫ��������������
��������ȡ������ɫ�����Һ���Թܣ��þƾ��Ƽ��ȣ���Һ�ָ���ɫ���������һ����
��������������������һ������һ��������жϣ������������£�
ij��ɫ��Һ�϶�û��MnO4������ɫ���п��ܺ���Na�����϶��У���Ϊֻ����һ�������ӣ���SO42�� ��MnO4���϶�û��MnO4������ɫ����
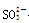 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�����pH��ֽ���飬��ҺpH��7��˵����Һ���ּ��ԣ������ڼ��Ի����в��ܴ�����������Ӳ��ܴ��ڣ��˴������ų��κ����ӣ�
������Һ�еμ���ˮ��������������ټ���CCl4������,CCl4��ʳȺ�ɫ���÷�Һ©����Һ��˵��һ����Br�����ӣ���Ϊ�嵥���������Ȼ�̼���dzȺ�ɫ�ģ�
�����Һ���ˮ��Һ�м���AgNO3��HNO3���Һ���а�ɫ�����������жϲ����Dz����������ӣ���Ϊǰ��ӵ�����ˮ�����к��������ӣ���ʵ�����и��ŵġ�ֻ��˵�����������ӡ�
����ȡԭ��Һ��������Ba(NO3)2������Ļ��Һ��������ɫ�������϶��������������Һ�е�������������Ӱ�����������������ˣ�����һ�������������������Һ�п϶����е�������Na+ ��Br����
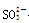
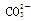 ���϶�û�е�������MnO4�� ��������е����ӷ���ʽ��Cl2+2Br��== Br2+2 Cl����
���϶�û�е�������MnO4�� ��������е����ӷ���ʽ��Cl2+2Br��== Br2+2 Cl����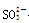 +Cl2+H2O ==2H++2Cl��+SO42����SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
+Cl2+H2O ==2H++2Cl��+SO42����SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��SO2��SO2��ˮ���õIJ���Ư���˺�ɫ����Һ��SO2��NaOH��Ӧ��������Һ��PH��
���ʵ��֤���ķ���������һ��ȡ������ɫ�����Һ���Թܣ�����NaOH��Һ����Һ�ָ���ɫ��������������
��������ȡ������ɫ�����Һ���Թܣ��þƾ��Ƽ��ȣ���Һ�ָ���ɫ���������һ������
�����������ƶ���ⷨ���ɣ���Щ�ƶ���Ľⷨ�����������ӵ����з�Ӧ�Լ����Ӽ�Ĺ���������ڽ���֮ǰ��Ӧ�����ṩ����������Һ���ܷ����������з�������������������һ����˵�����Ӽ������ɳ����������塢��������ʣ��Լ��ܷ���������ԭ��Ӧ�ģ��Ͳ�������Һ�д������档���磬H+��OH- ��H+������������ӣ�OH- �����������ӣ�Ag+��Cl- ��Br- ��I- ��
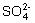 ��
��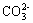 ��Ba2+��Ca2+��
��Ba2+��Ca2+��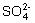 ��
��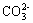 ��Fe2+��
��Fe2+�� (����������)��Fe3+��S2- ��Al3+��
(����������)��Fe3+��S2- ��Al3+�� ��S2- �ȵȣ�����������Һ�й��档
��S2- �ȵȣ�����������Һ�й��档�ھ����ƶϹ����У�Ҫע�����¼��㣺
(1) ���ƶ����ӵĿ϶�����ڽ���������ǣ���Ҫ����һ��
(2) �ƶϹ����У�ǰ��Ľ��۲�Ӧ��ì�ܡ���ˣ�ǰ�����½��۵����ӣ��ں�����ƶϹ����пɲ����������ڷ����з���ǰ�������ì�ܣ���Ӧ�ҳ�����ԭ��
(3) �����ƶϽ��ʱ��Ӧ�ÿ����������棬���϶����ڵ����ӣ��϶������ڵ����ӣ������ж������������ӡ��������������������Ӧ�ǻ�������ģ��κ�һ������ֻ�ܳ���һ�Σ������ظ����֡���Ȼ�е���Ŀ�в�һ�������������Ҫ�ش𣬵���������ʱ��Ӧ�ÿ��ǵ���

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
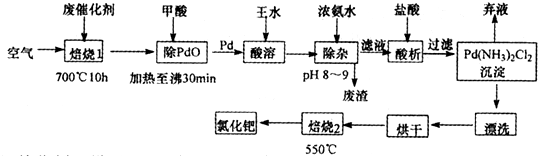
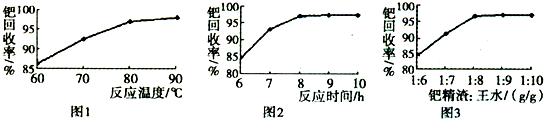
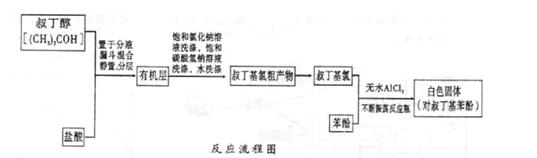
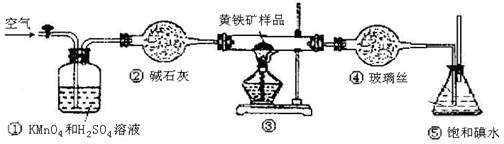
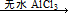 ArR+HX ��H<0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�����е�90.70C)�����ᷴӦ�Ƶ��嶡���ȣ��е�500C)��������Fridel��Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�990C)����Ӧ���̼�ʵ��װ������ͼ��ʾ��
ArR+HX ��H<0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�����е�90.70C)�����ᷴӦ�Ƶ��嶡���ȣ��е�500C)��������Fridel��Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�990C)����Ӧ���̼�ʵ��װ������ͼ��ʾ��