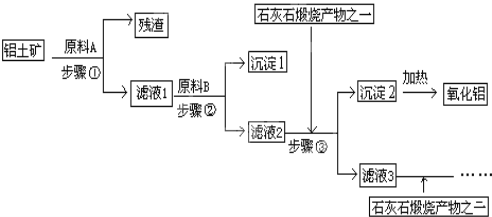
��Ŀ����
����Ŀ��25��ʱ����0.1molL-1NaOH��Һ�ζ�ij��Ԫ����H2A��H2A���ζ�������pH�����ֲַ�������[��(X)=![]() ]��ͼ��ʾ��
]��ͼ��ʾ��

����˵���������( )
A.��NaOH��Һ�ζ�0.1molL-1NaHA��Һ���÷�̪��ָʾ��
B.0.1molL-1NaHA��Һ�У�c(Na+)��c(HA-)��c(H2A)��c(A2-)
C.0.1molL-1Na2A��Һ�У�c(Na+)��c(HA-)+2c(A2-)
D.H2A��Ka2=1��10-7
���𰸡�B
��������
��ͼ��֪��ʵ��Ϊ0.1molL1 NaOH��Һ�ζ���Ԫ����H2A�ĵζ����ߣ�����Ϊ0.1molL1NaOH��Һ�ζ���Ԫ����H2A�����ֲַ��������ߡ���![]() ��1ʱ����Ӧ����NaHA��NaHA��Һ�����ԣ���
��1ʱ����Ӧ����NaHA��NaHA��Һ�����ԣ���![]() ��2ʱ����Ӧ����Na2A��Na2A��Һ�Լ��ԣ��Դ˽����⡣
��2ʱ����Ӧ����Na2A��Na2A��Һ�Լ��ԣ��Դ˽����⡣
A.ǿ��ζ�����ʱ���ζ��յ㣬��Һ�ʼ��ԣ�Ӧѡ�÷�̪��ָʾ��������NaOH��Һ�ζ�0.1molL1NaHA��ҺӦ�÷�̪��ָʾ������A��ȷ��
B.��ͼ��֪����![]() ��1ʱ����Ӧ����NaHA��NaHA��Һ�����ԣ�˵��HA�ĵ���̶ȴ���ˮ��̶ȣ�����Һ��c(A2)��c(H2A)����B����
��1ʱ����Ӧ����NaHA��NaHA��Һ�����ԣ�˵��HA�ĵ���̶ȴ���ˮ��̶ȣ�����Һ��c(A2)��c(H2A)����B����
C.0.1molL1Na2A��Һ�д��ڵ���غ��ϵc(Na��)��c(H��)��c(HA)��2c(A2)��c(OH)����Na2A��Һ�Լ��ԣ�c(OH)��c(H��)����c(Na��)��c(HA)��2c(A2)����C��ȷ��
D.��ͼ��֪������(X)Ϊ50%ʱ����Һ��c(HA)��c(A2)��pH��7����Ka2��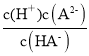 ��c(H��)��1��107����D��ȷ��
��c(H��)��1��107����D��ȷ��
��ѡ��B��

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�����Ŀ��һ���¶��£������������Ϊ 1.0L�ĺ����ܱ������з�����Ӧ��2CH3OH(g) CH3OCH3(g) + H2O(g) ����˵����ȷ���ǣ� ��
���� ��� | �¶�(��) | ��ʼ���ʵ���(mol) | ƽ�����ʵ���(mol) | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
I | 387 | 0.20 | 0.080 | 0.080 |
II | 387 | 0.40 | ||
III | 207 | 0.20 | 0.090 | 0.090 |
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. �ﵽƽ��ʱ������I�е�CH3OH�������������II�е�С
C. ����I�з�Ӧ����ƽ������ʱ�������III�ij�
D. ����ʼʱ������I�г���0.15mol ��CH3OH��0.15mol ��CH3OCH3��0.10mol ��H2O����Ӧ��������Ӧ�������