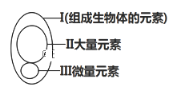
��Ŀ����
����Ŀ����ͼ��ʾϸ����ijЩ�л����Ԫ����ɺ��ܣ�����A��B����Ԫ�أ������������ӣ�ͼ��X��Y��Z��P�ֱ�Ϊ�����������ӵĻ�����λ����ش��������⣺

��1������������__________Ϊ�Ǽܣ����ǵĻ�����λ������Ϊ���壬����X������Ϊ��������ȼ�ϡ�������_______________������С����������Ҫ��ָ_______________��
��2��A��ʾ��Ԫ����__________��Y��Z����ɳɷ��ϵ������ǣ�Z���еijɷ���_________�� _________________�������ƣ���ϸ���к��м��A��C��T�ĺ�������________�֡�
��3�����ṹ�����Ե�ԭ���P������_____________________________________________������P���ɸ����ںϳɺ���3�����Ģ��Ĺ����й�����200��ˮ���ӣ���P����ĿΪ ______����
��4��Ⱦɫ��(�壩��Ҫ��_________��__________��ɣ�����ĸ����Ⱦɫ����Ⱦɫ��Ĺ�ϵ�ǣ�___________________________________________________________________��
���𰸡�̼�� ������ ���� N��P ���� ����� 5 �����ᣨP�����ࡢ��Ŀ������˳��ͬ 203 �� �� ͬ����������ϸ����ͬʱ�ڵ����ִ���״̬
��������
��ͼ�������������������Դ���ʣ���ʾ���࣬���Ԫ��ΪC��H��O���������Ƕ��ǵĻ�����ɵ�λ����С���еĶ�����Ҫ�ǵ��ۡ�
����Ҫ�ֲ���ϸ�����У���ʾDNA��������λY��ʾ�������������Ҫ�ֲ���ϸ�����У���ʾRNA��������λZ��ʾ���Ǻ�������Ԫ��ΪC��H��O��N��P�����̿�ʹDNA������ɫ������ʹRNA�ʺ�ɫ��
����������ijе��ߣ���ʾ�����ʣ�������λP�ǰ�������Ԫ������ΪC��H��O��N��
��1������������̼��Ϊ�Ǽܣ����ǵĻ�����λ������Ϊ���壬����X������Ϊ��������ȼ�ϡ������������ǣ��������������Դ���ʣ���С����������Ҫ��ָ���ۡ�
��2��C��H��O��A���DNA��RNA��DNA��RNA��C��H��O��N��P����Ԫ����ɣ���A��ʾ��Ԫ����N��P��Y�����������Z���Ǻ����ᣬ��������ɳɷ��ϵ������ǣ�Z���еijɷ��Ǻ��Ǻ� ����ण�ϸ���м���DNAҲ��RNA����ϸ���к��м��A��C��T�ĺ�������5�֡�
��3������ʾ�����ʣ��ṹ�����Ե�ԭ���P�������η�������Ϊ�����ᣨP�����ࡢ��Ŀ������˳��ͬ�����������-������=�ļ���=��ȥ��ˮ����������
����P���ɸ����ںϳɺ���3���������Ĺ����й�����200��ˮ���ӣ���P����ĿΪ 200+3=203����
��4��Ⱦɫ��(�壩��Ҫ������DNA�������������ʣ���ɣ�Ⱦɫ����Ⱦɫ��Ĺ�ϵ�ǣ�ͬ����������ϸ����ͬʱ�ڵ����ִ���״̬��

����Ŀ����֪���ᾧ��(H2C2O4��XH2O)������ˮ�����������Ը��������Һ��ȫ��Ӧ:2KMnO4+5H2C2O4+3H2SO4==K2SO4+2MnSO4+10CO2��+8H2O������������ԭ�ζ����ⶨ���ᾧ��Ľᾧˮ������X����������:
���÷�����ƽ��ȡ���ᾧ��1.260g���������Ƴ�100.00mL���������Һ
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊ0.1000mol/L��KMnO4����Һ���еζ������ν������:
��һ�εζ� | �ڶ��εζ� | �����εζ� | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪H2C2O4����Է�������Ϊ90����ش���������:
��1���ζ�ʱ��KMnO4����ҺӦ��װ��________(����ʽ���ʽ)�ζ����С�
��2��������ʵ�������,����Ҫ����������Ʒ��______(�����)��
��100mL����ƿ���ձ��۵ζ��ܼТ�©���ݲ�������������ƽ
��3������ζ��յ�ı�־��____________________________________________��
��4�������������ݼ���X=________________��
��5��������(��ƫ�ߡ�ƫ�ͻ���Ӱ��):
�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵ________��
����KMnO4����ҺŨ��ƫ�ͣ���Xֵ_________��
������ƿϴ����ƿ�ڻ���������������ˮ����Xֵ____________��