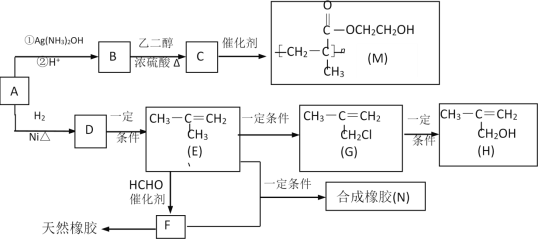
��Ŀ����
����Ŀ���о�NO2��SO2 ��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1��һ�������£���2molNO��2molO2���ں����ܱ������з������·�Ӧ��2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
A.��ϵѹǿ���ֲ���
B.���������ɫ���ֲ���
C.NO��O2�����ʵ���֮�ȱ��ֲ���
D.ÿ����1 molO2ͬʱ����2 molNO
��2��CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����ƽ����ø����Ũ�ȣ�
CH3OH(g)����ƽ����ø����Ũ�ȣ�
���� | CO | H2 | CH3OH |
Ũ�ȣ�mol/L�� | 0.9 | 1.0 | 0.6 |
�ش��������⣺
�ٻ�������ƽ����Է�������=_________________��
��ƽ�ⳣ��K=__________________��
�������������ѹ��Ϊ1L���������㣬Ԥ����ƽ����c(H2)��ȡֵ��Χ��__________��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH����ʱv��______v�������������������=������
���𰸡�A��B��C��D 18.56 0.67 1mol/L��c(H2)��2mol/L =
��������
��1��A.�÷�Ӧ���ߵĻ�ѧ����������ȣ��ڷ�Ӧû�дﵽƽ��ʱ����������ʵ����ᷢ���ı䣬��ϵ��ѹǿҲҪ�ı䣬���ѹǿ����˵�����������������������ȣ���Ӧ�ﵽ��ƽ�⣬A����ȷ��
B.��Ӧ��NO2����ɫ���壬��ɫ����˵��NO2��Ũ�Ȳ��ٸı䣬��Ӧ�ﵽ��ƽ�⣬B����ȷ��
C.NO��O2����ʼ���ʵ�����ȣ�����ѧ��������ͬ���仯������ͬ�����û�дﵽƽ�⣬NO��O2�����ʵ���֮�Ȼᷢ���ı䣬�������ı�˵���ﵽ��ƽ�⣬C����ȷ��
D. ÿ����1 molO2ͬʱ����2 molNO�����淴Ӧ������ȣ�˵����Ӧ�ﵽ��ƽ�⣬D����ȷ��
��ѡABCD��
��2�����ɱ�������֪��CO��H2��CH3OH�����ʵ����ֱ���1.8mol��2mol��1.2mol�������ֱ�Ϊ1.8mol��28g/mol=50.4g��2mol��2g/mol=4g��1.2mol��32g/mol=38.4g�����������ƽ����Է�����������![]() =
=![]() =18.56g/mol�����ƽ����Է���������18.56��
=18.56g/mol�����ƽ����Է���������18.56��
��ƽ�ⳣ��K= ![]() =
=![]() ��0.67��
��0.67��
�������������ѹ��Ϊ1L����˲��H2��Ũ�ȱ�Ϊ2mol/L��ѹ������ʹ��ѹǿ����CO(g)+2H2(g)![]() CH3OH(g)�еĻ�ѧ������֪��ƽ�����ƣ�������Ũ�ȱ�С��������������ԭ��֪��ƽ��ʱ��������Ũ�ȷ�ΧΪ1mol/L��c(H2)��2mol/L��
CH3OH(g)�еĻ�ѧ������֪��ƽ�����ƣ�������Ũ�ȱ�С��������������ԭ��֪��ƽ��ʱ��������Ũ�ȷ�ΧΪ1mol/L��c(H2)��2mol/L��
�ܸ���������ݣ�����λmol/L��
CO | 2H2 | CH3OH | |
ԭƽ������Ũ�� | 0.9 | 1.0 | 0.6 |
�ٳ���Ũ�� | 0.3 | 0 | 0.2 |
���������Ũ�� | 1.2 | 1.0 | 0.8 |
Qc=![]() =
=![]() =0.67=K��˵����ʱ��ѧ��Ӧ�Դ�ƽ��״̬����v��=v����
=0.67=K��˵����ʱ��ѧ��Ӧ�Դ�ƽ��״̬����v��=v����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�