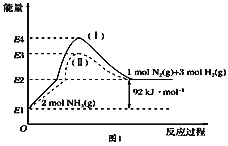
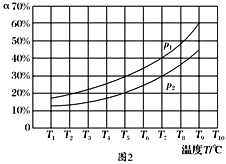
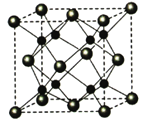
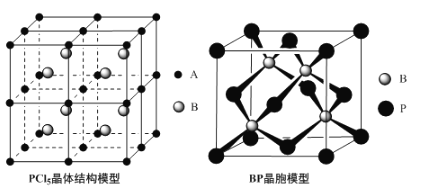
��Ŀ����
����Ŀ��ij��Һ�п��ܺ���K+��NH4+��Ba2+��SO42-��I-��Cl-��NO3-�еļ��֣�������Һ�ֳ����ȷݡ���������ʵ�飺��AgClʽ��Ϊ143.5��AgIʽ��Ϊ235��
����һ����Һ�м�������NaOH�����ȣ����ռ�����״̬�µ�����1.12L��
������һ����Һ�м�������Ba(NO3)2��Һ���а�ɫ�������������˵õ�����2.33g��
���ڢڵ���Һ�м�������AgNO3��Һ������4.7g����������
�йظ���Һ���������ࣨ������H+��OH-�����ж���ȷ����
A.��Һ��������2��������
B.ֻ��ȷ����Һ��NH4+��SO42-�Ƿ����
C.��Һ�������4��������
D.��Һ�в�����ͬʱ����K+��NO3-
���𰸡�C
��������
����һ����Һ�м�������NaOH�����ȣ����ռ�����״̬�µ�����1.12L������NaOH��Һ���Ȳ���������ֻ���ǰ�������һ������![]() �������ʵ���Ϊ0.05mol��
�������ʵ���Ϊ0.05mol��
������һ����Һ�м�������Ba(NO3)2��Һ���а�ɫ�������������˵õ�����2.33g����һ������![]() ��һ��������Ba2+����
��һ��������Ba2+����![]() ���ʵ���Ϊ��
���ʵ���Ϊ��![]() =0.01mol��
=0.01mol��
���ڢڵ���Һ�м�������AgNO3��Һ������4.7g�����������˳���δ˵����ɫ��������ֻ��AgCl����![]() ��������ֻ��AgI����
��������ֻ��AgI����![]() ������Һ�в�����K+��
������Һ�в�����K+��![]() �����ݵ���غ��֪��I��Cl�����ڣ�����Һ�в�����K+������
�����ݵ���غ��֪��I��Cl�����ڣ�����Һ�в�����K+������![]() ʱ��I��Cl���ܾ����ڣ�Ҳ����ֻI-������Һ�к���K+��������
ʱ��I��Cl���ܾ����ڣ�Ҳ����ֻI-������Һ�к���K+��������![]() ʱ��I��Cl���ܾ����ڣ�Ҳ����ֻCl-��������ֻ����I-��
ʱ��I��Cl���ܾ����ڣ�Ҳ����ֻCl-��������ֻ����I-��
A�����ݷ�����֪����Һ�п���ֻ����笠����ӣ���A����
B�����ݷ�����֪����Һ��һ������![]() ��
��![]() ������Cl��I�е�һ�ֻ�2�֣���B����
������Cl��I�е�һ�ֻ�2�֣���B����
C�����ݷ�����֪����Һ��![]() һ�����ڣ���Cl��I����1�ֻ�2�֣�
һ�����ڣ���Cl��I����1�ֻ�2�֣�![]() Ҳ���ܴ��ڣ���C��ȷ��
Ҳ���ܴ��ڣ���C��ȷ��
D��������������֪����Һ�п���ͬʱ����K+��![]() ����D����
����D����
�ʴ�Ϊ��C��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�