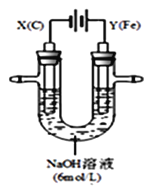
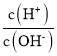ЬтФПФкШн
ЁОЬтФПЁПШчЭМЫљЪОЃЌдкЫЎВл![]() жазАга
жазАга![]() ЫЎЃЌШнЛ§ЮЊ
ЫЎЃЌШнЛ§ЮЊ![]() ЕФЪдЙм
ЕФЪдЙм![]() жаГфТњСЫ
жаГфТњСЫ![]() КЭ
КЭ![]() ЕФЛьКЯЦјЬх(БъзМзДПіЯТ)ЃЌНЋЪдЙм
ЕФЛьКЯЦјЬх(БъзМзДПіЯТ)ЃЌНЋЪдЙм![]() ЕЙВхШыЫЎВл
ЕЙВхШыЫЎВл![]() жаЁЃГфЗжЗДгІКѓЃЌЪдЙм
жаЁЃГфЗжЗДгІКѓЃЌЪдЙм![]() жаЪЃгрЦјЬхЕФЬхЛ§ЮЊ
жаЪЃгрЦјЬхЕФЬхЛ§ЮЊ![]() ЁЃ
ЁЃ
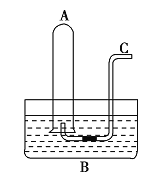
(1)НЋ![]() ЕЙВхШыЫЎВл
ЕЙВхШыЫЎВл![]() жаЗЂЩњЕФЗДгІЮЊ________________________________________________ЃЌИУЗДгІжабѕЛЏМСгыЛЙдМСЕФжЪСПБШЮЊ_____________ЃЛдЛьКЯЦјЬхжа
жаЗЂЩњЕФЗДгІЮЊ________________________________________________ЃЌИУЗДгІжабѕЛЏМСгыЛЙдМСЕФжЪСПБШЮЊ_____________ЃЛдЛьКЯЦјЬхжа![]() гы
гы![]() ЕФЮяжЪЕФСПжЎБШЮЊ____________ЁЃ
ЕФЮяжЪЕФСПжЎБШЮЊ____________ЁЃ
(2)ЭЈЙ§ЕМЦјЙм![]() ЯђЪЃгр
ЯђЪЃгр![]() ЦјЬхЕФЪдЙм
ЦјЬхЕФЪдЙм![]() жаГжајЭЈШыбѕЦјЃЌ
жаГжајЭЈШыбѕЦјЃЌ![]() жаПЩФмЙлВьЕНЕФЯжЯѓЪЧ_____________________________________________________________________________________________________ЁЃ
жаПЩФмЙлВьЕНЕФЯжЯѓЪЧ_____________________________________________________________________________________________________ЁЃ
(3)ЕБЪдЙм![]() жаГфТњЦјЬхЪБЭЃжЙЭЈШыбѕЦјЃЌШЛКѓНЋЪдЙмДгЫЎВлжаШЁГіЃЌЫЎВл
жаГфТњЦјЬхЪБЭЃжЙЭЈШыбѕЦјЃЌШЛКѓНЋЪдЙмДгЫЎВлжаШЁГіЃЌЫЎВл![]() жаШмвКЕФЮяжЪЕФСПХЈЖШЮЊ______
жаШмвКЕФЮяжЪЕФСПХЈЖШЮЊ______![]() (ЩшЫЎВлжавКЬхЕФЬхЛ§ШдЮЊ
(ЩшЫЎВлжавКЬхЕФЬхЛ§ШдЮЊ![]() )ЁЃ
)ЁЃ
ЁОД№АИЁП![]()
![]()
![]() ЮоЩЋЦјЬхБфЮЊКьзиЩЋЃЌЪдЙмжавКУцВЛЖЯЩЯЩ§жБжСШЋГфТњЃЌМЬајЭЈШыбѕЦјЃЌЪдЙмжавКЯТНЕЃЌзюКѓГфТњЮоЩЋЦјЬх
ЮоЩЋЦјЬхБфЮЊКьзиЩЋЃЌЪдЙмжавКУцВЛЖЯЩЯЩ§жБжСШЋГфТњЃЌМЬајЭЈШыбѕЦјЃЌЪдЙмжавКЯТНЕЃЌзюКѓГфТњЮоЩЋЦјЬх ![]()
ЁОНтЮіЁП
(1) ![]() гыЛсЫЎЗЂЩњЗДгІ
гыЛсЫЎЗЂЩњЗДгІ![]() ЃЛАДЛЏКЯМлБфЛЏЙцТЩевГібѕЛЏМСЁЂЛЙдМСВЂеЙПЊМЦЫуЃЛ
ЃЛАДЛЏКЯМлБфЛЏЙцТЩевГібѕЛЏМСЁЂЛЙдМСВЂеЙПЊМЦЫуЃЛ
(2)ЗДгІКѓЪдЙмФкЪЃгрЦјЬхЪЧNOЃЌЭЈШыбѕЦјКѓгыжЎЗДгІЃЌЩњГЩКьзиЩЋ![]() ЃЌвђДЫЗЂЩњ
ЃЌвђДЫЗЂЩњ![]() гыЫЎЕФЗДгІдйДЮЩњГЩNOЃЌжБЕНЯћКФЭъЕЊЕФбѕЛЏЮяЃЌОнДЫаДЗДгІЯжЯѓЃЛ
гыЫЎЕФЗДгІдйДЮЩњГЩNOЃЌжБЕНЯћКФЭъЕЊЕФбѕЛЏЮяЃЌОнДЫаДЗДгІЯжЯѓЃЛ
(3)МйЩшЫЎВлжавКЬхЕФЬхЛ§ШдЮЊ![]() ЃЌЧѓШмвКЕФЮяжЪЕФСПХЈЖШЃЌЙиМќдкгкИљОнЕЊдзгЪиКу
ЃЌЧѓШмвКЕФЮяжЪЕФСПХЈЖШЃЌЙиМќдкгкИљОнЕЊдзгЪиКу![]() ЃЛ
ЃЛ
(1)ЪдЙм![]() ЕЙВхШыЫЎВл
ЕЙВхШыЫЎВл![]() жаЃЌNOВЛгыЫЎЗДгІЃЌ
жаЃЌNOВЛгыЫЎЗДгІЃЌ![]() гыЫЎЗЂЩњЗДгІ
гыЫЎЗЂЩњЗДгІ![]() ЃЛД№АИЮЊЃК
ЃЛД№АИЮЊЃК![]() ЃЛ
ЃЛ
![]() жаЃЌбѕЛЏМСгыЛЙдМСОљЮЊ
жаЃЌбѕЛЏМСгыЛЙдМСОљЮЊ![]() ЃЌ
ЃЌ![]() зЊБфГЩЯѕЫсЪБзїЛЙдМСЃЌзЊБфГЩNOЪБзїбѕЛЏМСЃЌжЪСПБШЮЊ1ЃК2ЃЛД№АИЮЊЃК1ЃК2ЃЛ
зЊБфГЩЯѕЫсЪБзїЛЙдМСЃЌзЊБфГЩNOЪБзїбѕЛЏМСЃЌжЪСПБШЮЊ1ЃК2ЃЛД№АИЮЊЃК1ЃК2ЃЛ
ИљОнЬтвтЕУ![]() ЃЌдђ
ЃЌдђ![]() ЃК
ЃК![]() ЃЛД№АИЮЊЃК
ЃЛД№АИЮЊЃК![]() ЃЛ
ЃЛ
(2)ЪдЙм![]() жаЪЃгрЕФ
жаЪЃгрЕФ![]() ЦјЬхЮЊ
ЦјЬхЮЊ![]() ЃЌЭЈШыбѕЦјКѓЃЌЗЂЩњЗДгІ
ЃЌЭЈШыбѕЦјКѓЃЌЗЂЩњЗДгІ![]() ЁЂв§ЗЂ
ЁЂв§ЗЂ![]() ЃЌПЩКЯВЂГЩ
ЃЌПЩКЯВЂГЩ![]() ЃЌЛђ
ЃЌЛђ![]() ЃЌОнДЫаДЗДгІЯжЯѓЃЛД№АИЮЊЃКЮоЩЋЦјЬхБфЮЊКьзиЩЋЃЌЪдЙмжавКУцВЛЖЯЩЯЩ§жБжСШЋГфТњЃЌМЬајЭЈШыбѕЦјЃЌЪдЙмжавКЯТНЕЃЌзюКѓГфТњЮоЩЋЦјЬхЃЛ
ЃЌОнДЫаДЗДгІЯжЯѓЃЛД№АИЮЊЃКЮоЩЋЦјЬхБфЮЊКьзиЩЋЃЌЪдЙмжавКУцВЛЖЯЩЯЩ§жБжСШЋГфТњЃЌМЬајЭЈШыбѕЦјЃЌЪдЙмжавКЯТНЕЃЌзюКѓГфТњЮоЩЋЦјЬхЃЛ
(3)ЪдЙмжаГфТњЦјЬхЪБЃЌИљОнЕЊдзгЪиКуПЩЕУ![]() ЃЌдђ
ЃЌдђ ЃЛД№АИЮЊЃК
ЃЛД№АИЮЊЃК![]() ЁЃ
ЁЃ

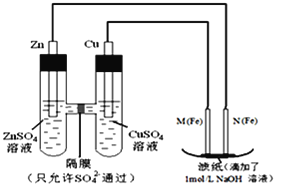
ЁОЬтФПЁПЭМАЦфЛЏКЯЮядкЙЄвЕЩњВњЩЯгааэЖргУЭОЁЃФГЙЄГЇвдЛдЭПѓ(жївЊГЩЗжЮЊ Cu2SЃЌКЌЩйСП Fe2O3ЁЂSiO2 ЕШдгжЪ)ЮЊдСЯжЦБИВЛШмгкЫЎЕФМюЪНЬМЫсЭЕФСїГЬШчЯТЃК

вбжЊЃК
ЂйГЃЮТЯТМИжжЮяжЪПЊЪМаЮГЩГСЕэгыЭъШЋГСЕэЪБЕФpHШчЯТБэ
Н№ЪєРызг | Fe2ЃЋ | Fe3ЃЋ | Cu2ЃЋ | Mn2ЃЋ |
ПЊЪМГСЕэ | 7.5 | 2.7 | 5.6 | 8.3 |
ЭъШЋГСЕэ | 9.0 | 3.7 | 6.7 | 9.8 |
Ђк Ksp[Fe(OH)3]ЃН4.0ЁС10Ѓ38
(1)МгПьЁАНўШЁЁБЫйТЪЃЌГ§ЪЪЕБдіМгСђЫсХЈЖШЭтЃЌЛЙПЩВЩШЁЕФДыЪЉга__________ЃЈШЮаДвЛжжЃЉЁЃ
(2)ТЫдќIжаЕФжївЊГЩЗжЪЧMnO2ЁЂSЁЂSiO2ЃЌЧыаДГіЁАНўШЁЁБЗДгІжаЩњГЩSЕФЛЏбЇЗНГЬЪНЃК______________ЁЃ
(3)ГЃЮТЯТЁАГ§ЬњЁБЪБМгШыЕФЪдМСAПЩгУCuOЕШЃЌЕїНкpHЕїЕФЗЖЮЇЮЊ_________ЃЌШєМг A КѓШмвКЕФ pHЕїЮЊ4.0ЃЌдђШмвКжа Fe3ЃЋЕФХЈЖШЮЊ_________mol/LЁЃ
(4)аДГіЁАГСУЬЁБ(Г§ Mn2ЃЋ)Й§ГЬжаЗДгІЕФРызгЗНГЬЪНЃК_________________________ЁЃ
(5)ЁАИЯАБЁБЪБЃЌзюЪЪвЫЕФВйзїЗНЗЈЪЧ________________ЁЃ
(6)Й§ТЫЂђЕУЕНЕФГСЕэОЙ§ЯДЕгЁЂИЩдяПЩвдЕУЕНМюЪНЬМЫсЭЃЌХаЖЯГСЕэЪЧЗёЯДОЛЕФВйзїЪЧ________________ЁЃ
ЁОЬтФПЁПвбжЊЗДгІX(g)ЃЋY(g)![]() R(g)ЃЋQ(g)ЕФЦНКтГЃЪ§гыЮТЖШЕФЙиЯЕШчБэЫљЪОЁЃ830 ЁцЪБЃЌЯђвЛИі2 LЕФУмБеШнЦїжаГфШы0.2 mol XКЭ0.8 mol YЃЌЗДгІГѕЪМ4 sФкv(X)ЃН0.005 mol/(LЁЄs)ЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
R(g)ЃЋQ(g)ЕФЦНКтГЃЪ§гыЮТЖШЕФЙиЯЕШчБэЫљЪОЁЃ830 ЁцЪБЃЌЯђвЛИі2 LЕФУмБеШнЦїжаГфШы0.2 mol XКЭ0.8 mol YЃЌЗДгІГѕЪМ4 sФкv(X)ЃН0.005 mol/(LЁЄs)ЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
ЮТЖШ/Ёц | 700 | 800 | 830 | 1 000 | 1 200 |
ЦНКтГЃЪ§ | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
A. 4 sЪБШнЦїФкc(Y)ЃН0.76 mol/L
B. 830 ЁцДяЦНКтЪБЃЌXЕФзЊЛЏТЪЮЊ80%
C. ЗДгІДяЦНКтКѓЃЌЩ§ИпЮТЖШЃЌЦНКте§ЯђвЦЖЏ
D. 1 200 ЁцЪБЗДгІR(g)ЃЋQ(g) ![]() X(g)ЃЋY(g)ЕФЦНКтГЃЪ§KЃН0.4
X(g)ЃЋY(g)ЕФЦНКтГЃЪ§KЃН0.4