��Ŀ����
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺��1����Ũ������HCL�����ʵ���Ũ��Ϊ
11.9
11.9
mol?L-1����2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����
BD
BD
������1��2���𰸣�A����Һ��HCL�����ʵ��� B����Һ��Ũ��
C����Һ��CL-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500ml ���ʵ���Ũ��Ϊ0.400mol?L-1��ϡ���ᣮ
�ٸ�ѧ����Ҫ��ȡ
16.8
16.8
ml ����Ũ����������ƣ��������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿������������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족����
I������Ͳ��ȡŨ��������ӹ۲찼Һ��
B
B
��II�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ
B
B
����������1������C=
=
=
���㣻
��2�����ݸ���ʽ�Ƿ�������й��жϣ�
��3���ٸ���C1V1=C2V2���㣻
�ڸ���C=
�жϣ�
| n |
| V |
| ||
| V |
| 103��w |
| M |
��2�����ݸ���ʽ�Ƿ�������й��жϣ�
��3���ٸ���C1V1=C2V2���㣻
�ڸ���C=
| n |
| V |
����⣺��1��C=
=
=
=
mol/L=11.9mol/L
�ʴ�Ϊ11.9mol/L��
��2��A��n=CV����������Һ����йأ���A�������⣮
B����Һ��Ũ���Ǿ�һ�ȶ��ģ�����ȡ��Һ������أ���B�������⣮
C��N=nNA=CVNA����������Һ����йأ���C�������⣮
D����Һ���ܶ��Ǿ�һ�ģ���������ȡ��Һ������أ���D�������⣮
��ѡ��BD��
��3����C1V1=C2V2��11.9mol/L��V1=0.400mol?L-1��0.5L������V1=0.0168L=16.8mL
�ʴ�Ϊ��16.8��
�ڸ���C=
�ж�
���ӹ۲찼Һ�棬��ȡ��Һ�����ƫ������Ũ��ƫС����ѡB��
����Һ�����ƫ������Ũ��ƫС����ѡB��
�ʴ�Ϊ��B�� B��
| n |
| V |
| ||
| V |
| 103��w |
| M |
| 103��1.19��36.5% |
| 36.5 |
�ʴ�Ϊ11.9mol/L��
��2��A��n=CV����������Һ����йأ���A�������⣮
B����Һ��Ũ���Ǿ�һ�ȶ��ģ�����ȡ��Һ������أ���B�������⣮
C��N=nNA=CVNA����������Һ����йأ���C�������⣮
D����Һ���ܶ��Ǿ�һ�ģ���������ȡ��Һ������أ���D�������⣮
��ѡ��BD��
��3����C1V1=C2V2��11.9mol/L��V1=0.400mol?L-1��0.5L������V1=0.0168L=16.8mL
�ʴ�Ϊ��16.8��
�ڸ���C=
| n |
| V |
���ӹ۲찼Һ�棬��ȡ��Һ�����ƫ������Ũ��ƫС����ѡB��
����Һ�����ƫ������Ũ��ƫС����ѡB��
�ʴ�Ϊ��B�� B��
���������⿼�������ʵ���Ũ�ȵ��йؼ��㼰����һ�����ʵ���Ũ�ȵ���Һ��֪ʶ�㣬�ѶȲ���Ҫע������һ�����ʵ���Ũ����Һ��������������C=
�жϣ������仯�����������Ӷ�ȷ��Ũ�ȵı仯��
| n |
| V |

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺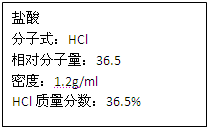 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺ ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺