��Ŀ����
��A��B��C��D����Ԫ�ء���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn��1npn��1�� Dԭ��M�ܲ�Ϊȫ����״̬������������ֻ��һ������ش��������⣺
��1��ACl3������A���ӻ�����Ϊ ��ACl3���ӵĿռ乹��Ϊ ��
��2��ijͬѧ����������Ϣ���ƶ�B�ĺ�������Ų�����ͼ��ʾ��
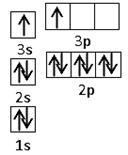
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��3��A��B��C��Ԫ��ԭ�ӵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����C60������ÿ��ԭ��ֻ�����ڵ�3��ԭ���γɹ��ۼ�����ÿ��ԭ������㶼����8�����ȶ��ṹ����C60�����Цм�����ĿΪ ��
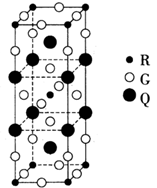
��4��D�Ļ�̬ԭ���� ��������ͬ�ĵ��ӣ�D2+ �ļ۵����Ų�ʽΪ ����֪D���������Ķѻ���ʽΪ���������ѻ���������һ�������ı߳�Ϊa cm����D������ܶ�Ϊ ��д����a�ı���ʽ����NA��ʾ�����ӵ�������ֵ����
��1��ACl3������A���ӻ�����Ϊ ��ACl3���ӵĿռ乹��Ϊ ��
��2��ijͬѧ����������Ϣ���ƶ�B�ĺ�������Ų�����ͼ��ʾ��

��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��3��A��B��C��Ԫ��ԭ�ӵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����C60������ÿ��ԭ��ֻ�����ڵ�3��ԭ���γɹ��ۼ�����ÿ��ԭ������㶼����8�����ȶ��ṹ����C60�����Цм�����ĿΪ ��
��4��D�Ļ�̬ԭ���� ��������ͬ�ĵ��ӣ�D2+ �ļ۵����Ų�ʽΪ ����֪D���������Ķѻ���ʽΪ���������ѻ���������һ�������ı߳�Ϊa cm����D������ܶ�Ϊ ��д����a�ı���ʽ����NA��ʾ�����ӵ�������ֵ����
��1��sp3�ӻ���2�֣� �����ͣ�2�֣�
��2���������ԭ����2�֣�
��3��N Si Mg ��2�֣� 30��2�֣�
(4) 7 ��1�֣� 3d9��1�֣�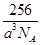 g/cm3��3�֣�
g/cm3��3�֣�
��2���������ԭ����2�֣�
��3��N Si Mg ��2�֣� 30��2�֣�
(4) 7 ��1�֣� 3d9��1�֣�
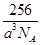 g/cm3��3�֣�
g/cm3��3�֣����������Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ���������Ų�Ϊns2np3��λ�����ڱ���VA�壬������̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������A��ΪN��B�Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ���������ֿ��ܣ���1s22s22p4��O����1s22s22p63s2��Mg����Oλ��p����Mgλ��s������BΪMg����s���ֻ�������������ӣ��ҵ����Ų�ʱ����s�������p�������n=2��C����Χ�����Ų�Ϊ3s23p2��CΪSi��Dԭ��M�ܲ�Ϊȫ����״̬������������ֻ��һ�������������Ų�Ϊ1s22s22p63s23p63d104s1����DΪ29��Ԫ��Cu��
��1��ACl3����ΪNCl3��������ԭ���ӻ������жϷ������÷��Ӽ۵��Ӷ�Ϊ(5+3)/2=4��������ԭ��Ϊsp3�ӻ�����N��һ���µ��Ӷԣ��ʷ��ӹ���Ϊ�����Ρ��÷��ӿɷ���NH3�����жϡ�
��2����ͬѧ�Ĵ�������3p����ϵĵ���Ӧ����3s����ϡ������������������͵Ĺ�����������ߵĹ�������������������ԭ����
��3��Ԫ�ص�һ�����ܣ�ͬ��������������С���ʵ�һ�����ܣ�N>P��ͬ�������������������ڶ�����������Ԫ�ش���ͬ���ڵ�������������Ԫ�ص�һ�����ܣ��ʵ�һ�����ܣ�P>Si>Mg���ۺϿ�֪����һ�������ɴ�С˳��ΪN>Si>Mg��C�������4�����ӣ�����3��������ԭ���γ�3�����ۼ�����ÿ��ԭ�����������8�������ȶ��ṹ������ÿ2��C���ṩһ�������γ�һ�����Ӷԣ��γɦм����ʦм�����Ϊ60��2=30����
��4��Cu�ĺ�������Ų�Ϊ1s22s22p63s23p63d104s1��������7��������ͬ�ĵ��ӣ�Cu2+��Cuʧȥ4s��3d����ϸ�һ�����ӣ���2�����ӣ����Լ۵����Ų�Ϊ3d9�����������ľ���ģ�����£�
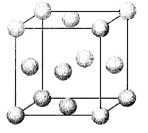
��ˣ�ÿ�������к��е�ԭ����Ϊ4����þ�����ܶ�Ϊ��
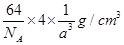 =
=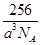 g/cm3��
g/cm3��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��
2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��



 ��ÿ�������з�̯2����ԭ��
��ÿ�������з�̯2����ԭ�� Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
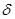 ����
����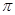 ���ĸ�����Ϊ ��
���ĸ�����Ϊ ��