��Ŀ����
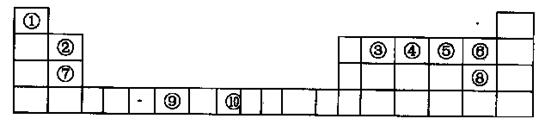
�±�Ϊ��ʽ���ڱ���һ���֣����е���Ŵ�����Ӧ��Ԫ�ء�
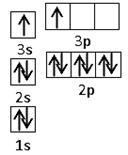
��1����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ �ӻ���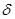 ����
����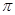 ���ĸ�����Ϊ ��
���ĸ�����Ϊ ��
��2��Ԫ�آۡ��ܡ��ݡ��ĵ�һ�������ɴ�С��˳��Ϊ �����Т�Ԫ�غ�����ӵĵ����Ų�ͼΪ ��
��3����Ԫ���γɵ�������������γɵľ����߳�Ϊacm�����侧���ܶ�Ϊ ��
��4��Ԫ�آܵ�����������Ӧ��ˮ����ϡ��Һ��Ԫ�آߵĵ��ʷ�Ӧʱ����ԭ����û�����壬�÷�Ӧ�����ӷ���ʽΪ ��
��5��Ԫ�آ�����γɵ�ԭ�Ӹ�����Ϊ1��1�����������ĵ����γ���ʽΪA��BC��5������������ﳣ����ΪҺ̬�������ڷǼ����ܼ����侧������ �����������һ�������·ֽ����ɢ�ĵ��ʺ͢�����γɵ�ԭ�Ӹ�����Ϊ1��1��������ڷֽ�������ƻ��Ļ�ѧ��Ϊ ���γɵĻ�ѧ��Ϊ ��
��6����ѧ����һ�ּ��⣬��Ϊͬ��Ԫ���γɵĺ������иó���Ԫ�صĻ��ϼ�Խ�ߣ�����Խǿ�����ñ��е�Ԫ�ؾ���˵����������ǿ������˳�� ��

��1����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ �ӻ���
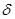 ����
����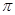 ���ĸ�����Ϊ ��
���ĸ�����Ϊ ����2��Ԫ�آۡ��ܡ��ݡ��ĵ�һ�������ɴ�С��˳��Ϊ �����Т�Ԫ�غ�����ӵĵ����Ų�ͼΪ ��
��3����Ԫ���γɵ�������������γɵľ����߳�Ϊacm�����侧���ܶ�Ϊ ��
��4��Ԫ�آܵ�����������Ӧ��ˮ����ϡ��Һ��Ԫ�آߵĵ��ʷ�Ӧʱ����ԭ����û�����壬�÷�Ӧ�����ӷ���ʽΪ ��
��5��Ԫ�آ�����γɵ�ԭ�Ӹ�����Ϊ1��1�����������ĵ����γ���ʽΪA��BC��5������������ﳣ����ΪҺ̬�������ڷǼ����ܼ����侧������ �����������һ�������·ֽ����ɢ�ĵ��ʺ͢�����γɵ�ԭ�Ӹ�����Ϊ1��1��������ڷֽ�������ƻ��Ļ�ѧ��Ϊ ���γɵĻ�ѧ��Ϊ ��
��6����ѧ����һ�ּ��⣬��Ϊͬ��Ԫ���γɵĺ������иó���Ԫ�صĻ��ϼ�Խ�ߣ�����Խǿ�����ñ��е�Ԫ�ؾ���˵����������ǿ������˳�� ��
��1��sp2����1�֣� 5��1��1�֣�
��2��F>N>O>C ��1�֣�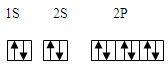 ��2�֣�
��2�֣�
��3��176/a3NA g/cm3��2�֣�
��4��4Mg+10H++NO3��=4Mg2++NH4++3H2O ��3�֣�
��5�����Ӿ��壻��1�֣���λ������1�֣���������1�֣�
��6��HNO3��HNO2����HClO4��HClO3��HClO2��HClO��д������Ҳ���֣���2�֣�
��2��F>N>O>C ��1�֣�
 ��2�֣�
��2�֣���3��176/a3NA g/cm3��2�֣�
��4��4Mg+10H++NO3��=4Mg2++NH4++3H2O ��3�֣�
��5�����Ӿ��壻��1�֣���λ������1�֣���������1�֣�
��6��HNO3��HNO2����HClO4��HClO3��HClO2��HClO��д������Ҳ���֣���2�֣�
���������ͼ��ΪԪ�����ڱ���ǰ������Ԫ�أ�����1����10˳��ֱ���H��Be��C��N��O��F��Mg��Cl��Cr��Fe��1��������γ���ϩ������̼Ԫ���γ�̼̼˫����sp2�ӻ�����2����һ������F��ǿ������Nԭ��p����ϰ�������Ƚ��ȶ�����Oǿ��Cԭ����С��F>N>O>C ����3��CO2�����������������壬������4�����ӣ��ܶ�Ϊ
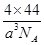 ����4��þ�����ᷢ��������ԭ��Ӧ�����������ɣ�˵�����ᱻ��ԭΪ��Σ���4Mg+10H++NO3��=4Mg2++NH4++3H2O��(5)A��BC��5��Fe(CO)5�� ������ﳣ����ΪҺ̬���۵�ͣ������ڷǼ����ܼ����Ƿ��Ӿ��塣
����4��þ�����ᷢ��������ԭ��Ӧ�����������ɣ�˵�����ᱻ��ԭΪ��Σ���4Mg+10H++NO3��=4Mg2++NH4++3H2O��(5)A��BC��5��Fe(CO)5�� ������ﳣ����ΪҺ̬���۵�ͣ������ڷǼ����ܼ����Ƿ��Ӿ��塣
��ϰ��ϵ�д�
�����Ŀ

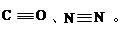 ��2��CO��N2�Ľṹ�ɱ�ʾΪ��
��2��CO��N2�Ľṹ�ɱ�ʾΪ�� 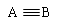
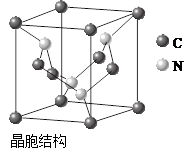

 ���������к��еĻ�ѧ�������� ������ĸ��ţ���
���������к��еĻ�ѧ�������� ������ĸ��ţ��� ��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����
��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����