��Ŀ����
���ܵĴ洢������Ӧ�õ���Ҫƿ����Ŀǰ�����û������о�����Ҫ��������У���λ�⻯��������廯���̼�ʲ��ϡ������⻯��ȡ�
��1��Ti��BH4��2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ�
��Ti2+��̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
��BH��4�Ŀռ乹���� ����������������
��2��Һ���Ǹ������ʣ������ܵ��������壬����N2+3H2 2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��
2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��
a.NH3������Nԭ�Ӳ���sp3�ӻ�
b.��ͬѹǿʱ��NH3�е��PH3��
c.[Cu(NH3)4]2+�����У�Nԭ������λԭ��
d.CN���ĵ���ʽΪ��
��3��2008�꣬Yoon���˷���Ca��C60���ɵ�Ca32C60�ܴ�������H2���ӡ�

��C60���������ڱ���CS2��˵��C60�� ���ӣ�ѡ������ԡ������Ǽ��ԡ�����
��1mol C60�����У����ЦҼ���ĿΪ ��
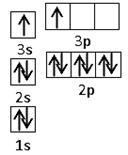
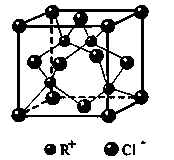
��4��MgH2�ǽ����⻯�ﴢ����ϣ��侧���ṹ��ͼ��ʾ����֪�þ�����ܶ�ag��cm-3���������Ϊ cm3[��a��NA��ʾ�����ӵ�����]��

��1��Ti��BH4��2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ�
��Ti2+��̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
��BH��4�Ŀռ乹���� ����������������
��2��Һ���Ǹ������ʣ������ܵ��������壬����N2+3H2
 2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��
2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��a.NH3������Nԭ�Ӳ���sp3�ӻ�
b.��ͬѹǿʱ��NH3�е��PH3��
c.[Cu(NH3)4]2+�����У�Nԭ������λԭ��
d.CN���ĵ���ʽΪ��

��3��2008�꣬Yoon���˷���Ca��C60���ɵ�Ca32C60�ܴ�������H2���ӡ�

��C60���������ڱ���CS2��˵��C60�� ���ӣ�ѡ������ԡ������Ǽ��ԡ�����
��1mol C60�����У����ЦҼ���ĿΪ ��
��4��MgH2�ǽ����⻯�ﴢ����ϣ��侧���ṹ��ͼ��ʾ����֪�þ�����ܶ�ag��cm-3���������Ϊ cm3[��a��NA��ʾ�����ӵ�����]��

��1����1s22s22p63s23p63d2����[Ar]3d2�� ����������
��2��abcd
(3) �ٷǼ��� ��90NA��5.418��1024
��4��52/aNA
�����������1��BH4-����ԭ���ӻ���ʽΪsp3���ʿռ乹��Ϊ�������壻��2��a��NH3������Nԭ�Ӽ۵��Ӷ���Ϊ��5+3��/2=4���ʲ���sp3�ӻ�����ȷ��b��NH3���Ӽ������������۷е�ϸߣ���ȷ��c��[Cu(NH3)4]2+�����У�Cu������ԭ�ӣ�Nԭ������λԭ�ӣ���ȷ��d��CN���뵪��Ϊ�ȵ����壬����ʽͬ�����ĵ���ʽ����ȷ����3�����������Ƿ��Ӿ�������ʣ����Ͷ���̼�ǷǼ��Է��ӣ�����Ϊ���Ӿ��壻���þ�̯������Ҽ���Ŀ��1��̼ԭ���γ�3���Ҽ���ÿ���Ҽ�2��̼ԭ�ӹ��ã�����60��3��1/2=90����4��������������������ٸ����ܶȼ����������������������к���MgH2����Ϊ2����Mg 8��1/8+1=2 H 4��1/2+2=4����ʽΪ2/NA=va/26 v=52/aNAcm3

��ϰ��ϵ�д�
�����Ŀ
 CH��C��N���Ʊ����ڵ�ԭ��,������ЦҼ��ͦм��ĸ���֮��Ϊ��������������������ȣ�,д���÷���������̼ԭ�ӵ��ӻ���ʽ����������
CH��C��N���Ʊ����ڵ�ԭ��,������ЦҼ��ͦм��ĸ���֮��Ϊ��������������������ȣ�,д���÷���������̼ԭ�ӵ��ӻ���ʽ����������  Ϊ̼ԭ��,
Ϊ̼ԭ��, Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ�����������������辧���߳�Ϊa cm,�ܶ�Ϊb g/cm3,���ӵ������ɱ�ʾΪ�����ú�a��b��ʽ�ӱ�ʾ����
Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ�����������������辧���߳�Ϊa cm,�ܶ�Ϊb g/cm3,���ӵ������ɱ�ʾΪ�����ú�a��b��ʽ�ӱ�ʾ���� 






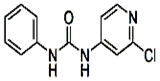
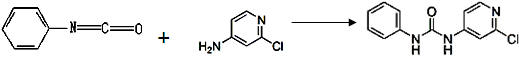


 ����Ӧ�漰���������У�X���� ������������(�� ��������)��MgO������۵��CaO�ߣ���Ҫԭ���������������������������������� ��
����Ӧ�漰���������У�X���� ������������(�� ��������)��MgO������۵��CaO�ߣ���Ҫԭ���������������������������������� ��