��Ŀ����
�±���Na��Mg��Si��S��Br����Ԫ�صĵ��ʵķе㣬����b��e�����Ⱥ͵�������塣
(1) a��Ԫ����Ԫ�����ڱ��е�λ��Ϊ________��
(2) д��d��Ԫ��ԭ�ӵĵ���ʽ________��������������γɵľ���Ϊ________����(������)������ǿ������ӷ���ʽΪ________________________________________��
(3) c�����γɵķ���X�Ŀռ乹��Ϊ________��д��X��ˮ��Һ������bԪ�ص�����������Ӧˮ���ﷴӦ�����ӷ���ʽ___________________________________��
(4) ����Ԫ�������γɵļ������а뾶��С����________�������ӷ��ţ�������������Ӧˮ����������ǿ����________(�����ʽ)��eԪ����NԪ���γɻ�����ĵ���ʽΪ____________________________��
(5) bԪ������Ԫ���γɵĻ�����Y�ľ����У�1�������ں��еĻ�������������________��
| ���� | a | b | c | d | e |
| �е�(��) | 58.8 | 882.9 | 444.7 | 2 355 | 1 107 |
(2) д��d��Ԫ��ԭ�ӵĵ���ʽ________��������������γɵľ���Ϊ________����(������)������ǿ������ӷ���ʽΪ________________________________________��
(3) c�����γɵķ���X�Ŀռ乹��Ϊ________��д��X��ˮ��Һ������bԪ�ص�����������Ӧˮ���ﷴӦ�����ӷ���ʽ___________________________________��
(4) ����Ԫ�������γɵļ������а뾶��С����________�������ӷ��ţ�������������Ӧˮ����������ǿ����________(�����ʽ)��eԪ����NԪ���γɻ�����ĵ���ʽΪ____________________________��
(5) bԪ������Ԫ���γɵĻ�����Y�ľ����У�1�������ں��еĻ�������������________��
��11�� ��ע����ÿ��1�֣���1���������ڵڢ�A��
��2��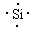 �� ԭ�ӣ� Si+2OH-+H2O��SiO32-+2H2��
�� ԭ�ӣ� Si+2OH-+H2O��SiO32-+2H2��
��3��V�� H2S+2OH-��S2-+H2O ��4��Mg2+ H2SO4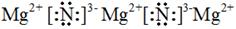
��5��4��Na+ 4��Cl-��2�֣�
��2��
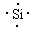 �� ԭ�ӣ� Si+2OH-+H2O��SiO32-+2H2��
�� ԭ�ӣ� Si+2OH-+H2O��SiO32-+2H2����3��V�� H2S+2OH-��S2-+H2O ��4��Mg2+ H2SO4
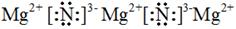
��5��4��Na+ 4��Cl-��2�֣�
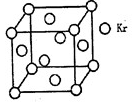
����������ƺ�þ�γɵľ����ǽ������壬�����Ӱ뾶����þ���ӱȽϣ����Խ���þ�ķе�����Ƶġ�b��e�����Ⱥ͵�������壬����b���ƣ�e��þ������ԭ�Ӿ��壬�е���ߣ�����d�ǹ衣S�͵������γɵľ�����Ƿ��Ӿ��壬����S���ʵķе���ڵ�����ģ�����a���壬c��S��
��1����λ��Ԫ�����ڱ��ĵ������ڵڢ�A�塣
��2����λ�ڵڶ���ȷ�ڢ�A�壬�����ʽ��
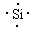 �����������γɵľ�����ԭ�Ӿ��塣���ʹ��ܺ�����������Һ��Ӧ����Ӧ�����ӷ���ʽ��Si+2OH-+H2O��SiO32-+2H2����
�����������γɵľ�����ԭ�Ӿ��塣���ʹ��ܺ�����������Һ��Ӧ����Ӧ�����ӷ���ʽ��Si+2OH-+H2O��SiO32-+2H2������3��H2S������Sԭ�Ӻ��еŶԵ��Ӷ�������6��2��1����2��2������H2S��V�νṹ��H2S�Ƕ�Ԫ���ᣬ���Ժ���������������Һ��Ӧ�����ӷ���ʽ��H2S+2OH-��S2-+H2O��
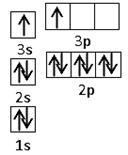
��4���ں�������Ų���ͬ�������£����뾶��ԭ���������������С�������γɵļ������а뾶��С����Mg2+���ǽ�����Խǿ�����������ˮ���������Խǿ����������������Ӧˮ����������ǿ��H2SO4����Ԫ�غ�þ�γɵĻ����ﵪ��þ�Ǻ������Ӽ������ӻ��������ʽ��
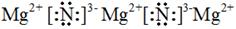 ��
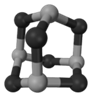
����5�������Ȼ��Ƶľ����ṹ��֪��1�������ں��еĻ�������������4��Na+��4��Cl-��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ������������ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
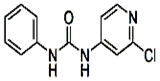
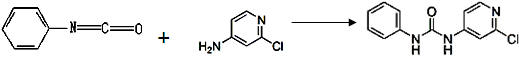
 �����Ȼ���Ļ�ѧʽΪ__________��
�����Ȼ���Ļ�ѧʽΪ__________��