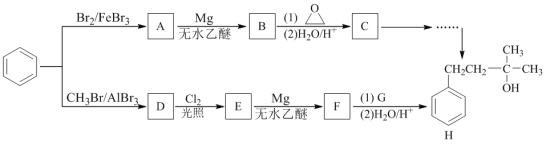
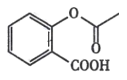
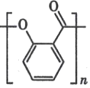
��Ŀ����
����Ŀ����Ѫ֬����Ӱ�����彡����������E��һ���ٴ����Ƹ�Ѫ֢֬��ҩ�E�ĺϳ�·�����£����ַ�Ӧ�������Լ��ԣ���
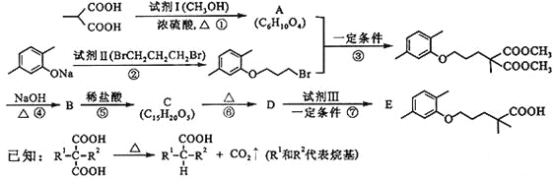
��ش��������⣺
��1���Լ�����������___a___���Լ����й����ŵ�������___b___������ ���ķ�Ӧ������____c___��
��2����������Ӧ�Ļ�ѧ����ʽ��_____________��
��3����������Ӧ�Ļ�ѧ����ʽ��_____________��
��4����������Ӧ�У��Լ���Ϊ�������������ṹ��ʽ��_________ ��
��5��C��ͬ���칹��������������ˮ�⣬����X��Y��CH3(CH2)4OH����X�����Ȼ��ͱ�������X��Y�ĺ˴Ź�������ֻ���������͵����շ壬��X��Y�������۷�Ӧ����������Ľṹ��ʽ��___________��
���𰸡���1��a�״� b��ԭ�� cȡ����Ӧ
��2��
��3��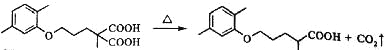
��4��CH3I
��5��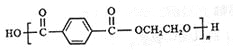
�������������������1���Լ����Ľṹ��ʽΪCH3OH������Ϊ�״����Լ����Ľṹ��ʽΪBrCH2CH2CH2Br�����������ŵ�����Ϊ��ԭ�ӣ�����![]() ��
��![]() �Ľṹ���Լ����жϵ������ķ�Ӧ����Ϊȡ����Ӧ��
�Ľṹ���Լ����жϵ������ķ�Ӧ����Ϊȡ����Ӧ��
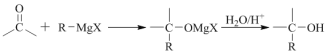
��2���������ת����ϵ֪��������ӦΪCH3CH(COOH)2��CH3OH��Ũ���ᡢ���ȵ������·���������Ӧ����CH3CH(COOCH3)2��ˮ����ѧ����ʽΪ ��
��
��3���������ת����ϵ�ƶ�CΪ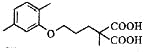 ����������Ϣ��Ӧ֪
����������Ϣ��Ӧ֪ �ڼ��������·�Ӧ����
�ڼ��������·�Ӧ����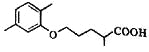 ����ѧ����ʽΪ
����ѧ����ʽΪ
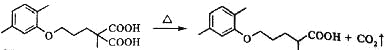 ��
��
��4���Լ���Ϊ���������������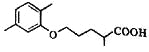 ��
��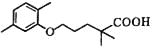 �Ľṹ�ƶϣ��Լ����Ľṹ��ʽ��CH3I��
�Ľṹ�ƶϣ��Լ����Ľṹ��ʽ��CH3I��
��5��C�ķ���ʽΪC15H20O5����ͬ���칹��������������ˮ�⣬��������������X��Y��CH3(CH2)4OH��������X�����Ȼ��ͱ�������X��Y�ĺ˴Ź�������ֻ���������͵����շ壬��XΪ�Զ������ᣬYΪCH2OHCH2OH����X��Y�������۷�Ӧ����������Ľṹ��ʽ��![]() ��
��