��Ŀ����
14�� ��28����ʵ��ʴ���ṩ��������ʾ�������д�������Ȼ��ˮ�������������1000�����Դ��Ҫ����Ȼ��ˮ�������־��壬������ƽ��ÿ46��ˮ���ӹ�����8������ÿ����������1��CH4���ӻ�1������H2O���ӣ�����������Ϣ������������⣺
��28����ʵ��ʴ���ṩ��������ʾ�������д�������Ȼ��ˮ�������������1000�����Դ��Ҫ����Ȼ��ˮ�������־��壬������ƽ��ÿ46��ˮ���ӹ�����8������ÿ����������1��CH4���ӻ�1������H2O���ӣ�����������Ϣ������������⣺��1�����й�����Ȼ��ˮ���������ַ���������ȷ����CE��
A�����ֶ��Ǽ��Է���
B�����ֶ��ǷǼ��Է���
C��H2O�Ǽ��Է��ӣ�CH4�ǷǼ��Է���
D��������ԭ�Ӻ�̼ԭ�ӵ��ӻ���ʽ�ֱ�Ϊsp2��sp3
E��������ԭ�Ӻ�̼ԭ�ӵ��ӻ���ʽ��Ϊsp3
��2��������ÿ8����ֻ��6��������CH4���ӣ�����2����������H2O������䣬����Ȼ��ˮ�����ƽ����ɿɱ�ʾΪCH4•8H2O��6CH4•48H2O��
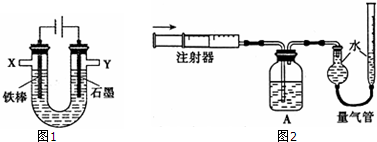
��A��B��C��D�Ƕ�����Ԫ�أ����ǵ����Ӿ�����ͬ�ĵ��Ӳ�ṹ���Ұ뾶���μ�С��A��D�Ļ�����X���������ᷴӦ��������NaOH��Һ��Ӧ��B��C��ɵ��͵����ӻ�����Y���侧��ṹ������NaCl���壻A��C��ԭ�Ӹ���֮��1��1��ɻ�����Z��
��3��д��Z�ĵ���ʽ
 ��
����4��CԪ����ɫ��Ӧ����ɫΪ��ɫ���Դ�ԭ�ӽṹ�ĽǶȽ�����ԭ����ȵ���������ԭ�ӵĺ�������������������Ӵӻ�̬ԾǨ������̬�����Ӵ��ڼ���̬���ȶ�������ԾǨ����̬���ڴ˹������ԹⲨ����ʽ�ͷ�������
��5��B��C��D��������ӻ�����CmDBn���侧��ṹ��Ԫ��ͼ��ʾ��������Cx+��o��ʾλ�������������е���������ڲ����ڲ���9��o��1��λ�����ģ���8��λ�ڴ������屻�ȷ�Ϊ8��С����������ģ���������DBnx-��•��ʾ��λ�ڸ�������Ķ�������ģ���x=1��m=3��n=6����֪�þ�����ܶ�Ϊag/cm3����þ����������Ӽ���������Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{840}{a{N}_{A}}}$��
���� I����1�����ӽṹ���Գƣ�������ɵ����IJ��غϵķ���Ϊ���Է��ӣ����ṹ�Գ���������ɵ������غϵķ���Ϊ�Ǽ��Է��ӣ����ݼ۲���Ӷ����ж��ӻ���ʽ��
��2��ÿ8������46��H2O���ӹ��ɿ�ܣ���������6��CH4���Ӻ�2��H2O���ӣ����ж�ÿ8������48��H2O���Ӻ�6��CH4���ӣ��ݴ��ƶϿɱ�ʾ��ȼ����ƽ����ɵĻ�ѧʽ��
��A��B��C��D�Ƕ�����Ԫ�أ����ǵ����Ӿ�����ͬ�ĵ��Ӳ�ṹ���Ұ뾶���μ�С��A��D�Ļ�����X���������ᷴӦ��������NaOH��Һ��Ӧ��X������������Al2O3��A�����Ӱ뾶����D����A��OԪ�ء�D��AlԪ�أ�B��C��ɵ��͵����ӻ�����Y���侧��ṹ������NaCl���壬B�����Ӱ뾶С��������BΪ�ǽ���Ԫ�ء�CΪ����Ԫ�أ���B��FԪ�ء�CΪNaԪ�أ�A��C��ԭ�Ӹ���֮��1��1��ɻ�����Z����Z��Na2O2���ݴ˽��
��� �⣺I��1��ˮ���ӵĿռ乹��ΪV�ͽṹ��������ɵ����IJ��غϣ����ڼ��Է��ӣ�
����Ŀռ乹��Ϊ�������壬�ṹ�Գƣ�������ɵ������غϣ����ڷǼ��Է��ӣ�
ˮ����������ԭ��O�ļ۲���Ӷ���=2+$\frac{6-1��2}{2}$=4������ԭ��Ϊsp3�ӻ���
����������ԭ��C�۲���Ӷ���=4+$\frac{4-1��4}{2}$=4����̼ԭ��Ϊsp3�ӻ���
��ѡ��CE��
��2��������ÿ8����ֻ��6��������CH4���ӣ�����2����������H2O������䣬���6��������6��������ӣ�ˮ����46+2=48����48��H2O������Ȼ��ˮ����ƽ��������ɿɱ�ʾΪCH4•8H2O��6CH4•48H2O��
�ʴ�Ϊ��CH4•8H2O��6CH4•48H2O��
��A��B��C��D�Ƕ�����Ԫ�أ����ǵ����Ӿ�����ͬ�ĵ��Ӳ�ṹ���Ұ뾶���μ�С��A��D�Ļ�����X���������ᷴӦ��������NaOH��Һ��Ӧ��X������������Al2O3��A�����Ӱ뾶����D����A��OԪ�ء�D��AlԪ�أ�B��C��ɵ��͵����ӻ�����Y���侧��ṹ������NaCl���壬B�����Ӱ뾶С��������BΪ�ǽ���Ԫ�ء�CΪ����Ԫ�أ���B��FԪ�ء�CΪNaԪ�أ�A��C��ԭ�Ӹ���֮��1��1��ɻ�����Z����Z��Na2O2��
��1��Z�ǹ������ƣ����������������Ӻ���������֮��������Ӽ���Oԭ��֮����ڹ��ۼ������������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��2��C��NaԪ�أ�����ɫ��ӦΪ��ɫ���ڼ��ȵ���������ԭ�ӵĺ�������������������Ӵӻ�̬ԾǨ������̬�����Ӵ��ڼ���̬���ȶ�������ԾǨ����̬���ڴ˹������ԹⲨ����ʽ�ͷ���������������ɫ��Ӧ�ʻ�ɫ��
�ʴ�Ϊ����ɫ���ڼ��ȵ���������ԭ�ӵĺ�������������������Ӵӻ�̬ԾǨ������̬�����Ӵ��ڼ���̬���ȶ�������ԾǨ����̬���ڴ˹������ԹⲨ����ʽ�ͷ�������
��3��C��NaԪ�أ������Ӵ�һ����λ����ɣ�����x=1���þ����������Ӹ���=9+12��$\frac{1}{4}$=12�������Ӹ���=6��$\frac{1}{2}$+8��$\frac{1}{8}$=4�����������ӡ������Ӹ���֮��=12��4=3��1������m=3�����ݻ��ϼ۵Ĵ�����Ϊ0֪��n=6��
����������Խ����ϵ�������֮��ľ���������辧���ⳤΪxcm������Խ��߳���Ϊ$\sqrt{3}$xcm����������������볤��Ϊ$\frac{\sqrt{3}}{4}$xcm���þ�����ܶ�Ϊa g/cm3���þ���ΪNa3AlF6����������Ϊ4��$\frac{23��3+17+19��6}{{N}_{A}}$g�������V=4��$\frac{23��3+17+19��6}{{N}_{A}}$g��a g/cm3=$\frac{840}{a{N}_{A}}$cm3���ʾ����ⳤ=$\root{3}{\frac{840}{a{N}_{A}}}$����������������볤��Ϊ$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{840}{a{N}_{A}}}$��
�ʴ�Ϊ��1��3��6��$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{840}{a{N}_{A}}}$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰���Ӽ��ԡ��ӻ�������������㡢��̬�뼤��̬��֪ʶ�㣬�ѵ��Ǿ������㣬�ؼ�����ȷ����������������ӣ���Ҫѧ������һ���Ŀռ���������ѧ������������Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ����Na2O2�����Һ�У�K+��AlO2-��NO3-��SO32- | |
| B�� | ˮ�����c��H+ ��=1��10-13 mol•L-1����Һ�У�Fe2+��Mg 2+��SO42-��NO3- | |
| C�� | ͨ������SO2�����Һ�У�Ba2+��Fe2+��H2SO3��Cl- | |
| D�� | 0.1mol•L-1 KMnO4������Һ�У�H2O2��NH4+��Br2��SO4 2- |
 ��ͼΪ��25mL0.1•L-1 NaOH��Һ����εμ�0.2mol•L-1 CH3COOH��Һ��������ҺpH�ı仯���ߣ�AB���䣬c��OH-����c��H+������c��OH-����c��CH3COO-����С��ϵ�ǣ�������
��ͼΪ��25mL0.1•L-1 NaOH��Һ����εμ�0.2mol•L-1 CH3COOH��Һ��������ҺpH�ı仯���ߣ�AB���䣬c��OH-����c��H+������c��OH-����c��CH3COO-����С��ϵ�ǣ�������| A�� | c��OH-�����ڡ�С�ڻ����c��CH3COO-�� | B�� | c��OH-��һ������c��CH3COO-�� | ||
| C�� | c��OH-��һ��С��c��CH3COO-�� | D�� | c��OH-��һ������c��CH3COO-�� |
| A�� | �õ���ܹ��ڸ����¹��� | |
| B�� | ��صĸ�����ӦΪ��C6H12O6+6H2O-24e-=6CO2��+24H+ | |
| C�� | �ŵ�����У�H+������������Ǩ�� | |
| D�� | �ڵ�ط�Ӧ�У�ÿ����1mol�����������������ɱ�״��������$\frac{2.42}{6}$L |
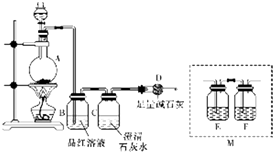 Ϊ̽��ij��̼�Ͻ���Ũ�����ڼ��������·�Ӧ�IJ��ֲ��ﲢ�ⶨ��̼�Ͻ�����Ԫ�ص�����������ij��ѧ�С���������ͼ��ʾ��ʵ��װ�ã����������ʵ��̽������Բ����ƿ�м���mg��̼�Ͻ𣬲��������Ũ���ᣬ��ȼ�ƾ��ƣ�
Ϊ̽��ij��̼�Ͻ���Ũ�����ڼ��������·�Ӧ�IJ��ֲ��ﲢ�ⶨ��̼�Ͻ�����Ԫ�ص�����������ij��ѧ�С���������ͼ��ʾ��ʵ��װ�ã����������ʵ��̽������Բ����ƿ�м���mg��̼�Ͻ𣬲��������Ũ���ᣬ��ȼ�ƾ��ƣ�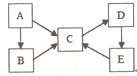 ����֪ʶ������ϵ��������֪ʶ�������Ӧ�ã���ͼA��B��C��D��E�������ʾ�����ͬһ��Ԫ�أ�����֮������ͼת����ϵ��
����֪ʶ������ϵ��������֪ʶ�������Ӧ�ã���ͼA��B��C��D��E�������ʾ�����ͬһ��Ԫ�أ�����֮������ͼת����ϵ�� ��
��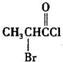 ��
��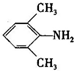 �Ǻϳ�ʩ������
�Ǻϳ�ʩ������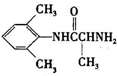 ��һ�ֿ�����ʧѧҩ����м��壬�ֱ�����ͼ��ʾ·�ߺϳɣ�
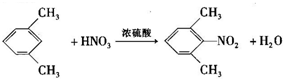
��һ�ֿ�����ʧѧҩ����м��壬�ֱ�����ͼ��ʾ·�ߺϳɣ�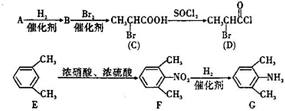 ����֪������-NH2�����ǰ�����-NH-�����м��ԣ�
����֪������-NH2�����ǰ�����-NH-�����м��ԣ�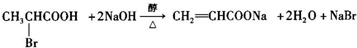 ��
�� ��
�� ��
��