题目内容
【题目】聚合氯化铝晶体是介于AlCl3和A1(OH)3之间的一种水溶性无机高分子聚合物,其制备原料主要是铝加工行业的废渣——铝灰,它主要含Al2O3、Al,还有SiO2等杂质。聚合氯化铝生产工艺流程如下:
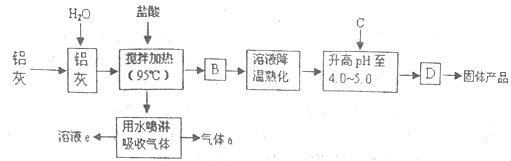
(1)反应中副产品a是______(用化学式表示);
(2)升高pH至4.0~4.5的目的是______;
(3)生产过程中可循环使用的物质是______(用化学式表示);
(4)为使得到的晶体较纯净,生产过程中使pH升高的C物质可选用______(填编号);
a.NaOHb.Alc.氨水d.A12O3e.NaAlO2
(5)聚合氯化铝晶体的化学式可表示为[A12(OH)nCl6-nH2O]m,实验室为测定n的值,进行如下操作:
①称取ag晶体,制成粉末,加热至质量不再变化时,得到bg。此过程可能用到的下列仪器有______;
a.蒸发皿 b.坩埚 c.研钵 d.试管
②另取a g晶体:用A试剂溶解→加足量AgNO3溶液→进行C操作→洗涤、烘干→称量为cg。则试剂A为______(填物质名称),C操作为______(填操作名称)。最后综合推算出n的值_____ 。
【答案】H2 促进AlCl3的水解,使晶体析出 HCl b、d b、c 硝酸 过滤 (861b-102c)/143.5b
【解析】
利用铝灰制备聚合氯化铝,先用盐酸溶解铝灰:Al2O3+6HCl=2AlCl3+3H2O,2Al+6HCl=2AlCl3+3H2↑,SiO2不溶于盐酸,调节AlCl3溶液的pH至4.0~5.0,使AlCl3部分水解,最后得到聚合三氯化铝。聚合氯化铝晶体{[A12(OH)nCl6-nH2O]m}的性质应类似于Al(OH)3和AlCl3。由此分析。
(1)铝灰中加盐酸搅拌加热至950C,有反应2Al+6HCl=2AlCl3+3H2↑,同时盐酸挥发出HCl气体,因此产生的H2中混有HCl气体,用水喷淋吸收气体中的HCl,所以副产品a是H2。
(2)因为聚合氯化铝晶体是介于AlCl3和A1(OH)3之间的物质,AlCl3在溶液中发生水解生成Al(OH)3:Al3++3H2O![]() Al(OH)3+3H+,升高溶液的pH至4.0~5.0的目的是:促进AlCl3的水解,使晶体析出。
Al(OH)3+3H+,升高溶液的pH至4.0~5.0的目的是:促进AlCl3的水解,使晶体析出。
(3)铝灰中加盐酸搅拌加热至950C,产生的H2中混有盐酸挥发出来的HCl气体,用水喷淋气体得到的溶液e即为稀盐酸溶液,盐酸可以循环使用。所以生产过程中可循环使用的物质的化学式为HCl。
(4)加入的c物质是为了促进AlCl3的水解反应:Al3++3H2O![]() Al(OH)3+3H+,Al和Al2O3都可以消耗H+而升高溶液的pH:2Al+6H+=2Al3++3H2↑,Al2O3+6H+=2Al3++3H2O,为不引入新的杂质,c物质应选用Al和Al2O3,答案选b、d。
Al(OH)3+3H+,Al和Al2O3都可以消耗H+而升高溶液的pH:2Al+6H+=2Al3++3H2↑,Al2O3+6H+=2Al3++3H2O,为不引入新的杂质,c物质应选用Al和Al2O3,答案选b、d。
(5)①晶体制成粉末要在研钵中将其研碎,加热固体物质一般使用坩埚,答案选bc。②该实验目的是测定a g晶体中Cl-的物质的量。溶解晶体的试剂应该选用硝酸溶液(既能溶解晶体也不干扰Cl-物质的量的测定),加入AgNO3溶液是为了将试样中的Cl-转化为AgCl沉淀,所以c操作是过滤,最后得到c g纯净的AgCl沉淀。c gAgCl的物质的量=![]() =
=![]() mol。聚合氯化铝晶体[A12(OH)nCl6-nH2O]m的性质应与Al(OH)3和AlCl3类似,所以加热晶体过程中先有AlCl3水解完全生成Al(OH)3,再有Al(OH)3受热分解,当加热至质量不再变化时,即得到b g纯净的Al2O3,因此a g晶体中铝元素物质的量=
mol。聚合氯化铝晶体[A12(OH)nCl6-nH2O]m的性质应与Al(OH)3和AlCl3类似,所以加热晶体过程中先有AlCl3水解完全生成Al(OH)3,再有Al(OH)3受热分解,当加热至质量不再变化时,即得到b g纯净的Al2O3,因此a g晶体中铝元素物质的量=![]() =
=![]() mol。所以聚合氯化铝晶体{[A12(OH)nCl6-nH2O]m}中铝元素与氯元素物质的量之比=2:(6-n)=
mol。所以聚合氯化铝晶体{[A12(OH)nCl6-nH2O]m}中铝元素与氯元素物质的量之比=2:(6-n)= ![]() :
:![]() ,解得n=
,解得n=![]() 。
。

 阅读快车系列答案
阅读快车系列答案【题目】利用含铜、铁的粗锌制备硫酸锌及相关物质。工艺流程图及有关数据如下:

物质 | Cu(OH)2 | Zn(OH)2 | Fe(OH)3 | ZnS | CuS |
Ksp | 5.0×10-20 | 2.0×10-16 | 4.0×10-38 | 1.2×10-23 | 8.5×10-45 |
请回答下列问题:
(1)粗锌中的铜与混酸的稀溶液反应的化学方程式为__________________________,图中处理气体X要能够体现绿色化学思想,还需补充气体______(填化学式)。
(2)若溶液I中c(Cu2+)为0.05mol·L-1,则溶液II中c(Fe3+)>____mol·L-1。
(3)若固体A是Zn,取9.61 g固体C溶解于足量的500mL 2 mol·L-1稀硝酸中,共收集到标准状况下2.24L的气体,向所得溶液中加入2 mol·L-1NaOH溶液,则当生成沉淀最多时,沉淀的质量为_____g;若固体A是另一种物质,取部分固体C于试管中,加入盐酸产生有臭鸡蛋味气体,则该反应的离子方程式为________________________。
(4)溶液Ⅲ经过蒸发浓缩、____________、过滤、洗涤、干燥,即得到较纯净的硫酸锌晶体;溶液还可以制备ZnS,实际选择的是(NH4)2S溶液而不是Na2S溶液作为反应物,理由是后者制得的ZnS含有较多的杂质,则该杂质是____________(填化学式)。
(5)金属锌常用作酸性干电池的负极,干电池不使用时,由于负极与电解质溶液接触而发生自放电反应:2NH4++Zn=2NH3+H2↑+Zn2+,造成电量自动减少。写出铅蓄电池不使用时,其正极上发生自放电的化学方程式__________________________。
【题目】(1)已知:Ti(s) +2Cl2(g) = TiCl4(l) △H = -804.2 kJ ·mol-1;
2Na(s) +Cl2(g) = 2NaCl(s) △H = -882.0 kJ ·mol-1
Na(s) = Na(l) △H =2.6 kJ ·mol-1
请写出用液态钠与四氯化钛置换出钛的热化学方程式__________________________。
(2)已知:向一个体积恒定为2L的密闭容器中充入4mol A、1mol B,发生如下反应:4A(g)+B(s) ![]() 3C(s)+4D(g)。该反应中各物质的摩尔质量(单位g·mol-1)都不一样,在一定条件下该反应2分钟达到平衡。
3C(s)+4D(g)。该反应中各物质的摩尔质量(单位g·mol-1)都不一样,在一定条件下该反应2分钟达到平衡。
①不能够说明该反应已达到平衡的是:________。
A.恒温下,容器内的压强不再变化
B.恒温下,容器内混合气体的密度不再变化
C.一定条件下,D的体积分数保持不变
D.一定条件下,单位时间内消耗4molA的同时生成1 mol B
②平衡后测得D的浓度为0.3mol·L-1,则从反应开始到平衡时,A的平均反应速率为________,B转化率为________。
(3)用活性炭还原法可以处理氮氧化物,有关反应为:2NO(g)+C(s) ![]() N2(g)+CO2(g);△H,向某密闭容器加入一定量的活性炭和NO,在t℃下反应,有关数据如图:
N2(g)+CO2(g);△H,向某密闭容器加入一定量的活性炭和NO,在t℃下反应,有关数据如图:
NO | N2 | CO2 | |
起始浓度/molL─1 | 0.10 | 0 | 0 |
平衡浓度/molL─1 | 0.04 | 0.03 | 0.03 |
①在t℃下,该反应的平衡常数为________(保留两位有效数字)。
②平衡后升高温度,再次达到平衡,测得容器中NO、N2、CO2的浓度之比为2:1:1,则该反应的
ΔH________0(填“>”、“<”或“=”),此时NO的转化率为________。