��Ŀ����
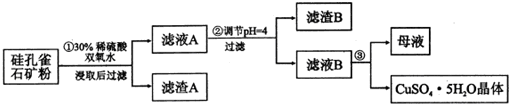
9�����ȸʯ��һ�ֺ�ͭ��ʯ����ͭ��̬ΪCuCO3•Cu��OH��2��CuSiO3•2H2O��ͬʱ����SiO2��FeCO3��Fe2O3��A12O3�����ʣ�����Ϊԭ����ȡ����ͭ�Ĺ���
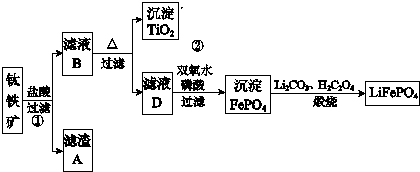
��������ͼ��ʾ��
��1������1���ȼ�������ϡ���ᣬ�ټ���˫��ˮ����˫��ˮ����÷�Ӧ�����ӷ���ʽ��2Fe2++H2O2+2H+�T2Fe3++2H2O
��2������ڵ�����ҺpH������ѡ�õ��Լ���BC��������ĸ��ţ�
A��Al2O3B��CuOC��CuCO3•Cu��OH��2
��3���й��������↑ʼ��������ȫ������pH���±���
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
��4������ҺBͨ������Ũ������ȴ�ᾧ�����˵Ȳ����ɵõ�����ͭ���壮
��5������ͭҲ������ͭ�������ڸ��¡����������»����Ƶã��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
ȡ384gCuS��һ�������º�������ȫ��Ӧ��������2CuS+3O2=2CuO+2SO2��
4CuS+5O2=2Cu2O+4SO2��������Ӧ�������ù�����Cu��O�����ʵ���֮��n��Cu����n��O��=4��a����ʱ���Ŀ��������ʵ���Ϊb mol����a=a=$\frac{2}{5}$b-8��������ռ���������1/5��
���� ���ȸʯ��һ�ֺ�ͭ��ʯ����ͭ��̬ΪCuCO3•Cu��OH��2��CuSiO3•2H2O��ͬʱ����SiO2��FeCO3��Fe2O3��A12O3�����ʣ����ȸʯ��ۼ���ϡ�����ܽ⣬����������������������ӽ�ȡ����ˣ��õ�������ҪΪSiO2��H2SiO3����ҺA�ں���Cu2+��Fe3+��Al3+��H+������ͭ�Ļ����������ҺPH=4ʹ������ȫ����������ʱ�������в��ֳ��������˵õ���ҺB��Ҫ������ͭ��Һ������������������������Һ��ͨ������Ũ����������ȴ�ᾧ�õ�����ͭ���壻
��1��˫��ˮ������Һ�н��������������������ӣ�
��2��������ҺA��ʾ���ԣ�������Լ����������µ����ʽ��з�����
��3�������������↑ʼ��������ȫ������pH�б����з���PH=4��ȫ����������ȫ���������ӣ�
��4����Һ�õ����ʾ���ķ���������Ũ������ȴ�ᾧ������ϴ�ӵõ�����ͭ���壻
��5����������ͭ��������Ӧ��������ͭ�����ݻ��ϼ������غ���ԭ�Ӹ����غ���ƽ����ʽ��
��CuO�����ʵ���Ϊx��Cu20�����ʵ���Ϊy��������ԭ�Ӹ����غ�������ɵ�CuO��Cu20��Ȼ�����n��Cu����n��O����
��� �⣺��1��˫��ˮ���������������������ӵ����ӷ���ʽΪ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
��2��������ҺA��ʾ���ԣ�������Լ��ܹ��к���Һ�е������ӣ������������µ����ʣ�����Ӧ��ѡ������ͭ����ʽ̼��ͭ��������ͭ���ȣ���ѡBC��
�ʴ�Ϊ��BC��
��3��������У�����pH=4ʱ����������B�ijɷ����ݱ������ݿ�֪��pH=4ʱ��������������ȫ������������������������������ȫ������Ҫ��PH��5.2������������û����ȫ������������������������������B�ijɷ�Ϊ��Fe��OH��3��Al��OH��3 ����ҺB�к��еĽ�����������Al3+��Cu2+��H+��
�ʴ�Ϊ��Al3+��Cu2+��H+��
��4����ҺBΪ����ͭ��Һ��ͨ������Ũ������ȴ�ᾧ������ϴ�ӵȲ����ɵõ�����ͭ���壻
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��5����������ͭ��������Ӧ��������ͭ�����ݻ��ϼ������غ���ԭ�Ӹ����غ���ƽ����ʽ��CuS+2O2$\frac{\underline{����}}{��}$CuSO4��
384g CuS���ʵ���=$\frac{384g}{94g/mol}$=4mol����CuO�����ʵ���Ϊx��Cu20�����ʵ���Ϊy�����ݷ���ʽ2CuS+302=2CuO+2S02��4CuS+502=2Cu20+4S02��
������ͭԭ�Ӹ����غ��֪��x+2y=4��
������ԭ�Ӹ����غ��֪��$\frac{1}{2}$x+$\frac{1}{2}$y+4=b��$\frac{1}{5}$��
��ã�x=0.8b-20��y=12-0.4b��
����n��Cu����n��O��=4��a=4����x+y��=4����$\frac{2}{5}$b-8����
����a=$\frac{2}{5}$b-8��
�ʴ�Ϊ��CuS+2O2$\frac{\underline{����}}{��}$CuSO4��a=$\frac{2}{5}$b-8��
���� ����Ϊ���������⣬�����˷���ʽ����д�����ʷ���ķ������йط���ʽ�ļ��㣬��Ҫ�������仯�������ʵķ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �պ��ľ̿���ȵ�Ũ���� | B�� | ͭ��Ũ���Ṳ�� | ||
| C�� | ʳ�κ�Ũ���Ṳ�� | D�� | �����е���Ũ���� |
| A�� | CH4��C2H4��C3H6��������� | |
| B�� | CH4��C3H6��C2H2����C3H6��C2H2=1��2�����ʵ���֮�ȣ� | |
| C�� | C2H6��C4H6��C2H2ͬ�����������Ϊ2��1��2 | |
| D�� | C3H8��C4H8��C2H2������Ϊ11��14��26 |
 �ζ�������һ�ֲ�����㡢ȷ�ȺܸߵĶ����������������ɹ㷺Ӧ�����к͵ζ���������ԭ��Ӧ�ȵζ��У�ij�о���ѧϰС���ͬѧ���õζ��������������������������
�ζ�������һ�ֲ�����㡢ȷ�ȺܸߵĶ����������������ɹ㷺Ӧ�����к͵ζ���������ԭ��Ӧ�ȵζ��У�ij�о���ѧϰС���ͬѧ���õζ����������������������������1���ⶨNaOH��Na2CO3�Ļ��Һ��NaOH�ĺ�����ʵ�����Ϊ��������Һ�мӹ�����BaCl2��ҺʹNa2CO3��ȫת����BaCO3������Ȼ���ñ�����ζ����÷�̪��ָʾ������
�������BaCO3������NaOH��Һ��ֱ�ӵ������ᣬ���յ���ɫ�ı仯Ϊ��Һ����ɫ�ɺ�ɫ��Ϊdz��ɫ��Ϊ�δ�������ܲ��NaOH�ĺ������ܣ���̪�ı�ɫ�ڼ��Է�Χ�ڣ���ʱֻ��NaOH��HCl��Ӧ��
�ڵζ�ʱ�����ζ����еĵζ�Һһֱ�½����������Ŵﵽ�ζ��յ㣬���ܷ��ɴ�ȷ�ؼ������������ܣ��ζ��ܵ��¶��̶ȣ����ζ�Һһֱ�½������������������Һ�����
��2���ⶨijƷ�Ƶĵ��Σ����е���أ��е�Ԫ�صİٷֺ�����ȷ��ȡ5.0000g�õ��Σ���������ˮ��Ȼ����������KI��Һ�����������»�ϣ������ķ�ӦΪKIO3+3H2SO4+5KI�T3K2SO4+3I2+3H2O������ַ�Ӧ�����Һϡ����250mL��Ȼ����5.0��10-4mol•L-1��Na2S2O3����Һ���еζ����õ�����ָʾ������ӦΪI2+
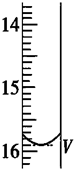
2S2O32-�T2I-+S4O62-����ȡ��Na2S2O3����ҺӦ���ü�ʽʽ�ζ��ܣ��й�ʵ����ֵ�������һ�εζ��յ��������ͼ��ʾ���뽫���õ�����������У���
| �ζ����� | ����Һ�������mL�� | �ζ�ǰ�Ķ�����mL�� | �ζ���Ķ�����mL�� |
| ��һ�� | 25.00 | 0.00 | V=15.90 |
| �ڶ��� | 25.00 | 0.00 | 14.99 |
| ������ | 25.00 | 0.00 | 15.01 |
a���ζ��յ�ʱ�����ӿ̶�
b��û����Na2S2O3����Һ��ϴ��Ӧ�ĵζ���
c����ƿ��������������ˮ��
| ���� | ���� |
| ��ʢ��4g��Na2O2���ձ��м���50mL����ˮ�õ���Һa | ���з�Ӧ��������ʹ������ľ����ȼ������ |
| ȡ5mL��Һa���Թ��У��������η�̪ | ������Һ��� ����10���ֺ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
��2��������Һ��ɫ��������Һa�д��ڽ϶��H2O2���̪�����˷�Ӧ��
��ͬѧͨ��ʵ��֤ʵ��H2O2�Ĵ��ڣ�ȡ������Һa�������Լ�MnO2���ѧʽ���������������
����ͬѧ�������ϻ�Ϥ����KMnO4���ԲⶨH2O2�ĺ�����ȡ15.00mL��Һa����ϡH2SO4�ữ����μ���
0.003mol•L-1��KMnO4��Һ���������壬��Һ��ɫ���ʿ�ʼ�������죬���յ�ʱ������20.00mL��KMnO4��Һ��
������ƽ��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O
����Һa��c��H2O2��=0.01mol•L-1��
����Һ��ɫ���ʿ�ʼ���������ԭ������Ƿ�Ӧ���ɵ�Mn2+��������
��3��Ϊ̽����������ԭ��ͬѧ�Ǽ�������������ʵ�飺
����H2O2��Һ�е������η�̪��������5��0.1mol•L-1 NaOH��Һ����Һ�����Ѹ�ٱ���ɫ�Ҳ������壬10���Ӻ���Һ����ɫ��
������0.1mol•L-1 NaOH��Һ�е������η�̪�ģ�����Һ��죬10���Ӻ���Һ��ɫ�����Ա仯�������Һ��ͨ��O2����Һ��ɫ�����Ա仯��
�ٴ�ʵ���͢��У��ɵó��Ľ����Ǽ��������£�H2O2�����̪��Ӧ��O2���ܣ�
��ͬѧ�ǽ�һ��ͨ��ʵ��֤ʵ����Һa�е����̪��H2O2���̪�����˻�ѧ��Ӧ��ʵ�鷽���ǣ�ȡ������Һa���Թ��У�����MnO2����ַ�Ӧ�����ϲ���Һ�е���2�η�̪���죬10���Ӻ���Һ��ɫ�����Ա仯��
| A�� | O2��O3 | B�� | ${\;}_{6}^{13}$C��${\;}_{6}^{12}$C | C�� | 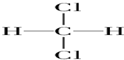 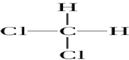 | D�� | CH3CH3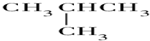 |