��Ŀ����
19��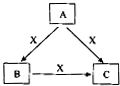 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ��Ҿ�Ϊ���壬ת����ϵ��ͼ��ʾ��ͼ�в��ֲ��P��������ȥ����A�Ĵ����˹��ϳ��ǻ�ѧ����������Խ����֮һ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ��Ҿ�Ϊ���壬ת����ϵ��ͼ��ʾ��ͼ�в��ֲ��P��������ȥ����A�Ĵ����˹��ϳ��ǻ�ѧ����������Խ����֮һ����ش���1��д��A�ķ���ʽNH3��
��2��д��A��X��Ӧ����C�Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��3�����ݻ�Ϊa mL���Թ��г���C���壬Ȼ������ˮ�У���ͨ��b mL X���壬��ַ�Ӧ�����Թ������ʣ������Ϊc mL����a��b��c�Ĺ�ϵ����Ϊ3��a-c��=4b��4��b-c��=3a��
���� ��ת����ϵ��֪A�к���Ԫ�ؾ��ж��ֻ��ϼۣ���ϡ�A�Ĵ����˹��ϳ��ǻ�ѧ����������Խ����֮һ����֪AΪNH3��BӦΪN2��CΪNO��XΪO2��NH3ȼ�տ�����N2���������¿�����NO��NO��������ˮ�пɷ���4NO+3O2+2H2O=4HNO3���Դ˽����⣮
��� �⣺��ת����ϵ��֪A�к���Ԫ�ؾ��ж��ֻ��ϼۣ���ϡ�A�Ĵ����˹��ϳ��ǻ�ѧ����������Խ����֮һ����֪AΪNH3��BӦΪN2��CΪNO��XΪO2��
��1�������Ϸ�����֪AΪNH3���ʴ�Ϊ��NH3��
��2�������ڴ���������������Ӧ����NO����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��3�����ݻ�Ϊa mL���Թ��г���NO���壬Ȼ������ˮ�У���ͨ��b mL O2���壬��ַ�Ӧ�����Թ������ʣ������Ϊc mL��ʣ���������ΪNO��Ҳ����Ϊ������
��ΪNO����4NO+3O2+2H2O=4HNO3���ɷ���ʽ��֪��Ӧ������V��NO����V��O2��=��a-c����b=4��3����3��a-c��=4b��
��ΪO2��������V��NO����V��O2��=a����b-c��=4��3����4��b-c��=3a��
�ʴ�Ϊ��3��a-c��=4b��4��b-c��=3a��
���� ���⿼�������ƶϣ�Ϊ�߿��������ͺ�Ƶ���㣬������Ԫ�ػ�����֪ʶ���ۺ���������õĿ��飬ע��������ʵ������Լ���Ӧ��ת���ص㣬��Ŀ����������ѧ���ķ����������������Ѷ��еȣ�

 53���ò�ϵ�д�
53���ò�ϵ�д�| A�� | �ӵ�ʳ�Σ���ʹ��������ɫ | |
| B�� | �ǽ���������һ��Ϊ���������� | |
| C�� | Ũ�����Ũ������ͭ��Ӧ���ܱ��ֳ�ǿ�����Ժ����� | |
| D�� | ʵ���ҿ����Ȼ�粒������������ƹ��干���ư��� |
| A�� | HF | B�� | HNO3 | C�� | NaCl | D�� | H2SO4 |
| A�� | H+��Fe3+��I-��SO42- | B�� | Al3+��Mg2+��HCO3-��Cl- | ||
| C�� | K+��Ca2+��NO3-��SiO32- | D�� | K+��Na+��OH-��AlO2- |
��

| A�� | �Ͽ�1mol O-O���ȶϿ�1mol N-N������������448kJ | |
| B�� | �Ͽ�1mol H-O����Ͽ�1mol H-N�������������Լ72.6kJ | |
| C�� | �����ϼ��ܵ���Ϣ��֪H2O�ķе��NH3�� | |
| D�� | ��Ԫ�طǽ����Ե�ǿ����֪H-O����H-N���� |
| A�� | �⻯����ȶ���Y��Z��W | |
| B�� | Y�ĵ��ʳ�����������ά | |
| C�� | W�ĵ��ʿ���Ϊˮ�����е������� | |
| D�� | X������������Ӧ��ˮ�������W�ĵ��ʷ�����Ӧ |
 �����ķֽⷴӦΪ��COCl2��g��?Cl2��g��+CO��g����H��0����Ӧ��ϵ��ƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ��������˵����ȷ���ǣ�������
�����ķֽⷴӦΪ��COCl2��g��?Cl2��g��+CO��g����H��0����Ӧ��ϵ��ƽ������ʵ�Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | �ӵ�10min����12minʱ������Cl2������v��Cl2��=0.01mol•L-1•min-1 | |
| B�� | ��8minʱ���18minʱ��Ӧ��ƽ�ⳣ����ͬ | |
| C�� | ����10minʱ�����ĸı䣬ʹCOCl2��ת���ʽ��� | |
| D�� | �ڵ�14minʱ���ı������������ѹ����Ӧ��������� |

| A�� | ���Ҵ���Ϊͬϵ�� | |
| B�� | һ���������������ᷢ��������Ӧ | |
| C�� | �������ڷ����廯�����ͬ���칹�� | |
| D�� | ������ˮ�����Ը��������Һ�����ӳɷ�Ӧ |
 ��-�Ȼ��������һ�ָ��ܵ�أ���ṹ��ͼ��ʾ���æ¡�-Al2O3�մɹ�Ϊ���壬����NaAlCl4Ϊ����ʣ��䷴ӦʽΪ��Ni+2NaCl$?_{�ŵ�}^{���}$2Na+NiCl2���õ�ر������й������ŵ籣�����ƣ����й��ڸõ�ص�����������ǣ�������
��-�Ȼ��������һ�ָ��ܵ�أ���ṹ��ͼ��ʾ���æ¡�-Al2O3�մɹ�Ϊ���壬����NaAlCl4Ϊ����ʣ��䷴ӦʽΪ��Ni+2NaCl$?_{�ŵ�}^{���}$2Na+NiCl2���õ�ر������й������ŵ籣�����ƣ����й��ڸõ�ص�����������ǣ�������| A�� | �õ���ڳ����²����������� | |
| B�� | ����NaAlCl4�����������缫��Ӧ | |
| C�� | ��������������Ӧ��NaCl+e-=Na+Cl- | |
| D�� | ���ŵ�ʱ������Ӧ��AlCl${\;}_{4}^{-}$-3e-=4Cl-+Al |