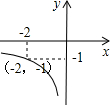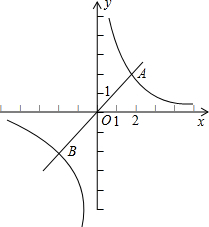ЬтФПФкШн
ШчЭМЃЌвЛДЮКЏЪ§y=kx+bЕФЭМЯѓгыЗДБШР§КЏЪ§y=
ЕФЭМЯѓЯрНЛгкAЁЂBСНЕуЃЎ
ЃЈ1ЃЉРћгУЭМжаЬѕМўЃЌЧѓЗДБШР§КЏЪ§гывЛДЮКЏЪ§ЕФЙиЯЕЪНЃЛ
ЃЈ2ЃЉИљОнЭМЯѓаДГіЪЙИУвЛДЮКЏЪ§ЕФжЕаЁгкИУЗДБШР§КЏЪ§ЕФжЕЕФxЕФШЁжЕЗЖЮЇЃЛ
ЃЈ3ЃЉЙ§BЕузїBHДЙжБгкxжсДЙзуЮЊHЃЌСЌНгOBЃЌдкxжсЪЧЗёДцдквЛЕуPЃЈВЛгыЕуOжиКЯЃЉЃЌЪЙЕУвдPЁЂBЁЂHЮЊЖЅЕуЕФШ§НЧаЮгыЁїBHOЯрЫЦЃПШєДцдкЃЌжБНгаДГіЕуPЕФзјБъЃЛВЛДцдкЃЌЫЕУїРэгЩЃЎ

| m |
| x |
ЃЈ1ЃЉРћгУЭМжаЬѕМўЃЌЧѓЗДБШР§КЏЪ§гывЛДЮКЏЪ§ЕФЙиЯЕЪНЃЛ
ЃЈ2ЃЉИљОнЭМЯѓаДГіЪЙИУвЛДЮКЏЪ§ЕФжЕаЁгкИУЗДБШР§КЏЪ§ЕФжЕЕФxЕФШЁжЕЗЖЮЇЃЛ
ЃЈ3ЃЉЙ§BЕузїBHДЙжБгкxжсДЙзуЮЊHЃЌСЌНгOBЃЌдкxжсЪЧЗёДцдквЛЕуPЃЈВЛгыЕуOжиКЯЃЉЃЌЪЙЕУвдPЁЂBЁЂHЮЊЖЅЕуЕФШ§НЧаЮгыЁїBHOЯрЫЦЃПШєДцдкЃЌжБНгаДГіЕуPЕФзјБъЃЛВЛДцдкЃЌЫЕУїРэгЩЃЎ

ЃЈ1ЃЉНЋAЃЈ-2ЃЌ1ЃЉДњШыЗДБШР§НтЮіЪНЕУЃК1=
ЃЌМДm=-2ЃЌ
ЁрЗДБШР§НтЮіЪНЮЊy=-
ЃЛ
НЋAЃЈ-2ЃЌ1ЃЉЃЌBЃЈ1ЃЌ-2ЃЉДњШыy=kx+bжаЕУЃК
ЃЌ
НтЕУЃК
ЃЌ
ЁрвЛДЮКЏЪ§НтЮіЪНЮЊy=-x-1ЃЛ
ЃЈ2ЃЉИљОнЭМЯѓЕУЃКвЛДЮКЏЪ§ЕФжЕаЁгкИУЗДБШР§КЏЪ§ЕФжЕЕФxЕФШЁжЕЗЖЮЇ-2ЃМxЃМ0ЛђxЃО1ЃЛ
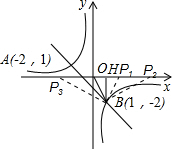
ЃЈ3ЃЉДцдкЃЌШчЭМЫљЪОЃК
ЕБЁїOBHЁеЁїP1BHЪБЃЌP1H=OH=1ЃЌМДOP1=2ЃЌP1ЃЈ2ЃЌ0ЃЉЃЛ
ЕБЁїOBHЁзЁїBP2HЪБЃЌЕУЕН
=
ЃЌМДHP2=
=
=4ЃЌМДOP2=OH+HP2=1+4=5ЃЌP2ЃЈ5ЃЌ0ЃЉЃЛ
ЕБЁїOBHЁзЁїBP3HЪБЃЌЕУЕН
=
ЃЌМДHP3=
=
=4ЃЌМДOP3=P3H-OH=4-1=3ЃЌP3ЃЈ-3ЃЌ0ЃЉЃЌ
злЩЯЃЌТњзуЬтвтPЕФзјБъЮЊЃЈ2ЃЌ0ЃЉЛђЃЈ5ЃЌ0ЃЉЛђЃЈ-3ЃЌ0ЃЉЃЎ
| m |
| -2 |
ЁрЗДБШР§НтЮіЪНЮЊy=-
| 2 |
| x |
НЋAЃЈ-2ЃЌ1ЃЉЃЌBЃЈ1ЃЌ-2ЃЉДњШыy=kx+bжаЕУЃК
|
НтЕУЃК
|
ЁрвЛДЮКЏЪ§НтЮіЪНЮЊy=-x-1ЃЛ
ЃЈ2ЃЉИљОнЭМЯѓЕУЃКвЛДЮКЏЪ§ЕФжЕаЁгкИУЗДБШР§КЏЪ§ЕФжЕЕФxЕФШЁжЕЗЖЮЇ-2ЃМxЃМ0ЛђxЃО1ЃЛ
ЃЈ3ЃЉДцдкЃЌШчЭМЫљЪОЃК
ЕБЁїOBHЁеЁїP1BHЪБЃЌP1H=OH=1ЃЌМДOP1=2ЃЌP1ЃЈ2ЃЌ0ЃЉЃЛ
ЕБЁїOBHЁзЁїBP2HЪБЃЌЕУЕН
| BH |
| OH |
| HP2 |
| HB |
| BH2 |
| OH |
| 4 |
| 1 |
ЕБЁїOBHЁзЁїBP3HЪБЃЌЕУЕН
| BH |
| OH |
| HP3 |
| BH |
| BH2 |
| OH |
| 4 |
| 1 |
злЩЯЃЌТњзуЬтвтPЕФзјБъЮЊЃЈ2ЃЌ0ЃЉЛђЃЈ5ЃЌ0ЃЉЛђЃЈ-3ЃЌ0ЃЉЃЎ


СЗЯАВсЯЕСаД№АИ
ЯрЙиЬтФП