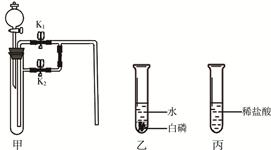
��Ŀ����
��ѧ�������ҵ�����������ÿ�����CO2�Ĺ��룺�ѿ������뱥��̼������Һ�У���Һ������CO2����̼�����ƣ�����̼�����ƹ����ַֽ�ų�CO2���ںϳ�����CO2��������Ӧ���ɼ״���CH3OH����ˮ����Ҫ��������������ͼ��ʾ��
[���Ͽ�Ƭ] ̼�����Ʒֽ��¶���270�棬̼������856���ۻ�������δ�ﵽ�ֽ��¶ȡ�
��ش��������⣺
��1�����ճ��з����˻��Ϸ�Ӧ����ѧ����ʽΪ ��
��2���������ÿ����е�CO2�����ʹ�����CO2Ũ�ȣ������ڼ��� ��
��3���ϳ����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4������������ѭ�����õ������� ���ѧʽ����
��1��Na2CO3+2HCl==2NaCl+H2O+CO2����2�֣���2������ЧӦ��1�֣�
��3��CO2+3H2 CH3OH+H2O��2�֣�
CH3OH+H2O��2�֣�
��4�� Na2CO3��H2O��2�֣�ÿ�ش�һ�֣�����һ�֣�
���������������1����ͼ�п�֪�������ճ���̼����������еĶ�����̼�����˷�Ӧ������̼�����ơ�����̼�����Ƶ����Ԫ�ؿ�֪����Ӧ�ﻹӦ�к���Ԫ�ص�����ˮ�����Է�Ӧ�Ļ�ѧ����ʽΪNa2CO3+2HCl==2NaCl+H2O+CO2����
��2��������̼���������ЧӦ����Ҫ���壬���Ի������ÿ����е�CO2�����ʹ�����CO2Ũ�ȣ������ڼ�������ЧӦ��
��3�����������֪���ںϳ����ڷ�����Ӧ�ķ�Ӧ��ΪCO2����������Ӧ����Ϊ ��������Ϊ�״���CH3OH����ˮ��
��������Ϊ�״���CH3OH����ˮ��
��4�������̵�Ŀ���ǹ�ҵ���������ÿ�����CO2��������ѭ�����õ�������Na2CO3��H2O��
���㣺̼������̼�����Ƶ����ʡ���ѧ����ʽ����д

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�ѧϰ�������Ƶ�����֮��֪������������Һ�к���H2O��Na+��OH������ʹ��ɫ��̪��Һ���ɫ��ij�Ƽ�С�����һ��̽������һ������ʹ��ɫ��̪��Һ���ɫ��
��������롿����٣�������H2O�� ����ڣ�������Na+�� ����ۣ�������OH����
��ʵ����֤��
| ʵ �� �� �� | ʵ �� �� �� | �� �� |
| �����Թ�ȡ��������ˮ������1-2����ɫ��̪��Һ���� | �Թ�����Һ��Ϊ��ɫ | ˮ���Ӳ���ʹ��ɫ��̪��Һ���ɫ |
| �����Թ�ȡ�����Ȼ�����Һ������1-2����ɫ��̪��Һ���� | | |
| �����Թ�ȡ��������������Һ������1-2����ɫ��̪��Һ���� | | |
�����۷�˼����ͬѧ��Ϊ����ٲ���Ҫʵ����֤�Ϳ����ų�������Ϊ��ͬѧ�������� ��
���л�ѧ���ϣ�ͬѧ����������ʵ��̽����Ļ�ѧ���ʣ�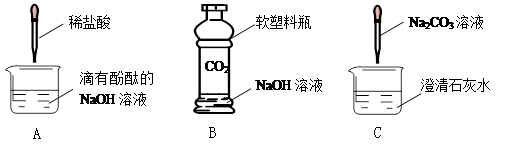
1��Aʵ���й۲쵽������Ϊ ��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
2��Bʵ���з�Ӧ�Ļ�ѧ����ʽΪ ��
3��Cʵ���й۲쵽�������� ��
4��ʵ�������ͬѧ�ǽ���Һ����ͬһֻ��Һ���У�����ַ�Һ���Dz��Ժ�ɫ��
��������⡿ ��Һ�к���ʲô���ʣ�
��������롿 ͨ����������ʵ�飬ͬѧ�Dz��룺��Һ�г���̪��̼��Ƽ�ˮ����һ������ ��
��ʦ�ʣ���Һ�л�������ʲô���ʣ�����ͬѧ���������ۣ������Ǵ�ҵķ��ԣ�
| ѧ���� | ������Ca(OH)2��NaOH | ѧ���� | ������Ca(OH)2��CaCl2 |
| ѧ���� | ������NaOH��Na2CO3 | ѧ���� | ������Na2CO3 |
| ѧ���� | ������NaOH | ���� | �������� |
��ʵ����֤�� ͬѧ�ǶԿ��ܺ��е����ʽ���ȷ����ȡһ�����ķ�Һ���ˣ�����Һ����μ����Ȼ�����Һ���õ���ɫ��������ɫ��ȥ��
�����ۡ�������Һ�У������� ��û�� ��
ij�о�С�鷢�֣�άC����Ƭ������ҩƷ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2������С��ͬѧ̽��������ijɷ֡�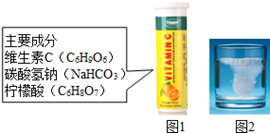
����������衿
С��˵�������������CO2��O2��CO��H2��N2��
С��˵�������ܺ���N2����Ϊ ��
С��˵�������ܺ���CO��H2����Ϊ��ҩƷ��ȫ�Ƕȿ��ǣ�H2��CO��ȼ�ױ���ͬʱCOҲ�� ����
��С��ͬѧ��Ϊ����������ܺ���CO2��O2�е�һ�ֻ����֡�
������ʵ�顿
| ʵ���� | ʵ����� | ʵ������ |
| �� | ������ͨ������ʯ��ˮ�� | ����ʯ��ˮ����� |
| �� | �������ǵ�ľ������������� | �����ǵ�ľ��û�и�ȼ |
��1����ʵ��ٿ�֪���������п϶������� ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����ʵ����� ����ܡ����ܡ���ȷ���������в��������������� ��
��ѧ�κ�ѧ��ȤС���ͬѧ������ʵ����ʱ��������һƿ����������Һû������Ƥ����������ʦͬ���չ������̽����
[�������1]������������Һ�Ƿ�������أ�
[ʵ��̽��1]
| ʵ����� | ʵ������ | ʵ����� |
| ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ������ð���� | ����������Һһ�������ˣ� |
[�������2]������������Һ��ȫ�����ʻ��Dz��ֱ����أ�
[���������]
����1������������Һ���ֱ��ʣ�
����2������������Һȫ�����ʣ�
[��������]
��1���Ȼ�����Һ�����ԣ�
��2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl
[ʵ��̽��2]
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | ���� �����ɣ� | ˵��ԭ��Һ��һ����̼���ƣ� |
| ��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ������ ���� |
[ʵ�����]������������Һ�� ��������֡���ȫ���������ʣ�
[��˼������]
��1������������Һ¶���ڿ��������ױ��ʣ���д����ط�Ӧ�Ļ�ѧ����ʽ���� ����
��2��������[ʵ��̽��2]�У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷�������������С������С�����
[������Ӧ��]
����������Һ���ױ��ʣ������ܷⱣ�森ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬������һ������ ����

 ���������ո��������̽�������������ʵ�飨��ͼ3��ʾ������ش�
���������ո��������̽�������������ʵ�飨��ͼ3��ʾ������ش�