��Ŀ����
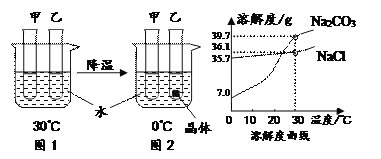
����Ŀ������� NaCl��NH4Cl �ڲ�ͬ�¶�ʱ���ܽ�ȡ�
�¶�/�� | 0 | 20 | 40 | 60 | 80 | |
�ܽ��/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | |
��1�������������ʵ��ܽ�����¶�Ӱ��ϴ����_____��
��2��40��ʱ���� 40.6g NaCl ���뵽 100g ˮ�У���ֽ���ʹ֮�ܽ⣬������Һ������Ϊ_____g��
��3��20��ʱ��NaCl ������Һ��������������Ϊ����ȷ��С����� 1 λ��_____��
���𰸡� NH4Cl�� 136.6 26.5%
��������
��1����NaCl��NH4Cl �ڲ�ͬ�¶�ʱ���ܽ�ȱ���֪���������ʵ��ܽ�����¶�Ӱ��ϴ����NH4Cl��
��2��40��ʱ��NaCl���ܽ����36.6g��������40��ʱ��100g��ˮ��������ܽ��Ȼ��Ƶ�����Ϊ36.6g���ʽ�40.6g NaCl ���뵽 100g ˮ�У���ֽ���ʹ֮�ܽ⣬������ܽ�36.6g����������Һ������Ϊ36.6g+100g=136.6g��
��3��������Һ��������������=![]() ��100%��20��ʱ��NaCl ������Һ��������������Ϊ
��100%��20��ʱ��NaCl ������Һ��������������Ϊ![]() ��100%��26.5%��
��100%��26.5%��

����Ŀ��Ϊ�ⶨij��ͭ��ͭп�Ͻ���Ʒ��ͭ�ĺ�����ij��ѧ�С������ν���ʵ�飬ʵ�������������ش��������⣺
��ȡҩƷ | ��һ�� | �ڶ��� | ������ |
��ͭ��Ʒ������g�� | 12 | 10 | 10 |
ϡ����������g�� | 100 | 100 | 150 |
��������������g�� | 0.2 | 0.2 | 0.2 |
��1����____��ʵ���У�ҩƷ�ɷ�ǡ����ȫ��Ӧ��
��2����ͭ��Ʒ��ͭ�����������Ƕ��٣�ǡ����ȫ��Ӧʱ������Һ���������������Ƕ���________������ȷ��0.1%��