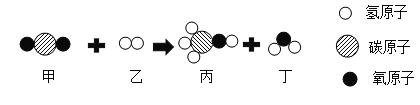
��Ŀ����
����Ŀ���������ͼ�ش��������
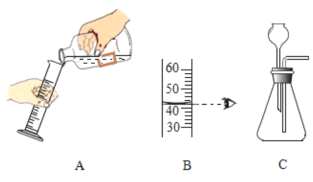
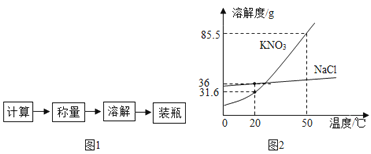
��1��A���㵹Һ��IJ����ǣ�ȡ��ƿ���������������ϣ���ǩ�������ģ���Ͳ����б����Ͳ����_____������������Ͳ�е���Һ�壻B��Һ��������_____mL��
��2����С��������ͼCװ�������壬���ָ�װ���д���_____������С�Ⱦ��������еĴ������������ſ���������ȡ��������ʵ������ȡ�����Ļ�ѧ����ʽΪ_____��
��С����ѡ���˲�ͬ�Ļ�ѧҩƷ���þ���������Cװ���Ƶ�����һ�����壬���������������ſ������ռ�����ʵ������ȡ������Ļ�ѧ����ʽΪ_____��
��Ϊȷ�����巢��װ�ã�Ӧ���Ƿ�Ӧ������_____��
���𰸡� ϸ��ƿ�ڽ����š��� 42�� 2 Zn+H2SO4=ZnSO4+H2������ CaCO3+2HCl=CaCl2+H2O+CO2������ ��Ӧ���״̬����
��������
��1��A���㵹Һ��IJ����ǣ�ȡ��ƿ���������������ϣ���ǩ�������ģ���Ͳ����б����Ͳ����ϸ��ƿ�����ţ�����������Ͳ�е���Һ�壻B��Һ��������42mL����2����С��������ͼCװ�������壬���ָ�װ���д���2����������©��Ӧ����Һ�����£�����Ӧ�ý�����Ƥ����������ʵ������ȡCO2�����ڳ����£��ô���ʯ��ʯ��ʯ��ϡ������ȡ�ģ�̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȡ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ�����Ϊȷ�����巢��װ�ã�Ӧ���Ƿ�Ӧ�����ͷ�Ӧ���״̬��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij������ȤС��Ϊ�˲ⶨ����ʯ��ʯ��CaCO3������������ȡ25gʯ��ʯ��Ʒ�������100gһ������������ϡ�����5�μ��뵽����Ʒ�У������������±���ʾ�����ʲ���ϡ���ᷴӦ��Ҳ������ˮ����
���� | һ | �� | �� | �� | �� |
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
�������������/g | 2.2 | 4.4 | 6.6 | 8.8 | 8.8 |
��1������CO2������Ϊ_________g��
��2����__________��ǡ����ȫ��Ӧ��
��3����ʯ��ʯ��Ʒ��CaCO3������������_________
����Ŀ������� NaCl��NH4Cl �ڲ�ͬ�¶�ʱ���ܽ�ȡ�
�¶�/�� | 0 | 20 | 40 | 60 | 80 | |
�ܽ��/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | |
��1�������������ʵ��ܽ�����¶�Ӱ��ϴ����_____��
��2��40��ʱ���� 40.6g NaCl ���뵽 100g ˮ�У���ֽ���ʹ֮�ܽ⣬������Һ������Ϊ_____g��
��3��20��ʱ��NaCl ������Һ��������������Ϊ����ȷ��С����� 1 λ��_____��