ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΆ≠ΦΑΆ≠ΒΡΜ·ΚœΈο‘Ύ…ζ≤ζΓΔ…ζΜν÷–”–Ή≈ΙψΖΚΒΡ”Π”ΟΓΘ
Θ®“ΜΘ©Ά≠ΒΡΙψΖΚ”Π”Ο
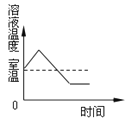
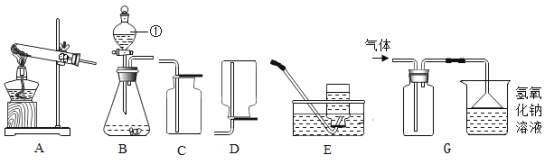
Θ®1Θ©Έ“Ιζ‘ΎΈςΚΚ ±ΤΎΨΆ”–ΓΑ‘χ«ύΒΟΧζ‘ρΜ·ΈΣΆ≠Γ±÷°ΥΒΘ§”ΟΜ·―ßΖΫ≥Χ Ϋ±μ ΨΤδ‘≠άμ______ΓΘ
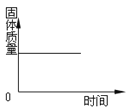
Θ®2Θ©Ά≠‘ΎΙΛ“Β…œΩ…”Ο”Ύ…ζ≤ζΒγά¬Θ®ΆβΟφΑϋΙϋΝΥΒγά¬ΤΛΘ©Θ§’β «άϊ”ΟΝΥΆ≠ΒΡ_____–‘ΓΘ
Θ®ΕΰΘ©ΝρΥαΆ≠ΨßΧεΒΡ÷Τ±Η
ΙΛ“Β…œ”ΟΚ§Ά≠ΖœΝœΘ®»γΘΚΥιΆ≠ΓΔΒγά¬ΤΛΒ»Θ©ΓΘΡ≥Μ·―ß–ΓΉιΒΡΆ§―ßΧα≥ωΩ…“‘”ΟΚ§Ά≠ΖœΝœ÷Τ±ΗΝρΥαΆ≠ΨßΧεΘ®CuSO4ΓΛxH2OΘ©Θ°÷ς“ΣΝς≥Χ»γœ¬ΘΚ
![]()
Θ®1Θ©Κ§Ά≠ΖœΝœΖέΥιΒΡΡΩΒΡ «__________ΓΘ
Θ®2Θ©ΗΟΝς≥Χ÷–Θ§Φ”»κH2O2Κσ“Σ«σΈ¬Ε»ΩΊ÷Τ‘Ύ50ΓφΒΫ60ΓφΦ”»»ΒΡ‘≠“ρΈΣ_____ΓΘ
Θ®3Θ©–Υ»Λ–ΓΉιΆ§―ß“Μ÷¬÷Η≥ωΘ§ΫΪ¬Υ“ΚΨ≠’τΖΔ≈®ΥθΓΔ_____Θ®ΧνΫαΨßΖΫΖ®Θ©ΓΔΙΐ¬ΥΒ»≤ΌΉςΚσΘ§”Ο…ΌΝΩ95%ΒΡΨΤΨΪΝήœ¥ΚσΝάΗ…Θ§ΒΟΝρΥαΆ≠ΨßΧεΘ®CuSO4ΓΛxH2OΘ©Θ§ΨßΧε≤…”ΟΨΤΨΪΝήœ¥Εχ≤Μ”ΟΥ°ΒΡ‘≠“ρ «____________ΓΘ
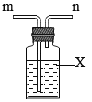
Θ®»ΐΘ©Ά≠…ζ–βΜα…ζ≥…Φν ΫΧΦΥαΆ≠Θ§ΕχΙΛ“Β…œΥυ÷ΤΒΟΒΡΦν ΫΧΦΥαΆ≠÷÷άύΫœΕύΘ§ΤδΉι≥…±μ ΨΈΣΘΚxCuCO3ΓΛyCu(OH)2ΓΛzH2OΓΘ
Ή ΝœΘΚxCuCO3ΓΛyCu(OH)2ΓΛzH2OΦ”»»÷Ν200 ±ΜαΖ÷ΫβΒΟΒΫ―θΜ·Ά≠Θ§Εΰ―θΜ·ΧΦΚΆΥ°ΓΘ
≤βΕ®Ρ≥÷÷≤ζΤΖΒΡΉι≥…Θ§ΗΟ–Υ»Λ–ΓΉιΉΦ»Ζ≥ΤΝΩΝΥ25.8gΒΡ―υΤΖΘ§…ηΦΤΝΥ»γœ¬ΉΑ÷Ο≤ΔΫχ–– Β―ιΘΚ
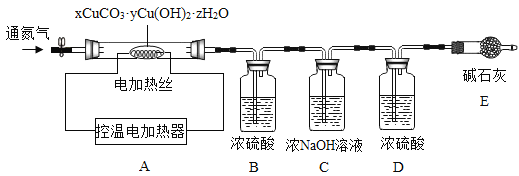
Β―ι ΐΨίΦ«¬Φ»γœ¬±μΘΚ
B÷–»ή“Κ÷ ΝΩ/g | C÷–»ή“Κ÷ ΝΩ/g | D÷–»ή“Κ÷ ΝΩ/g | E÷–ΙΧΧε÷ ΝΩ/g | |
Ζ¥”Π«Α | 100.0 | 100.0 | 100.0 | 120.5 |
Ζ¥”ΠΚσ | 105.4 | 104.4 | 100.2 | 120.5 |
Β―ιΖ÷ΈωΚΆ ΐΨί¥Πάμ
Θ®1Θ© Β―ι«Α”ΠΗΟœ»_________Θ§‘ΌΙΡ»κ“ΜΕΈ ±ΦδΒΣΤχΚσ≥ΤΝΩBΓΔCΓΔDΓΔEΒΡ÷ ΝΩΓΘ
Θ®2Θ©ΆΘ÷ΙΙΡ»κΒΣΤχΘ§ΒςΩΊΈ¬ΒγΦ”»»220Γφ≥÷–χΦ”»»Θ§Ιέ≤λΒΫΉΑ÷ΟB÷–______ ±±μΟςΦν ΫΧΦΥαΆ≠“―Ψ≠Άξ»ΪΖ÷ΫβΓΘ
Θ®3Θ©Ζ¥”ΠΫα χΚσΘ§‘ΌΜΚΜΚΙΡ»κ“ΜΕΈ ±ΦδΒΣΤχΒΡΡΩΒΡ «___________ΓΘ
Θ®4Θ©ΗΟΦν ΫΧΦΥαΆ≠ΒΡΜ·―ß Ϋ «__________ΓΘ
ΓΨ¥πΑΗΓΩFe+CuSO4=FeSO4+Cu ΒΦΒγ ‘ω¥σΖ¥”ΠΈο÷°ΦδΒΡΫ”¥ΞΟφΜΐΘ§Φ”ΩλΖ¥”ΠΥΌ¬ Ζά÷ΙΈ¬Ε»ΙΐΗΏΙΐ―θΜ·«βΖ÷Ϋβ ά以ΫαΨß Ζά÷ΙΨßΧε»ή”ΎΥ°¥χά¥ΥπΚΡΜρΨΤΨΪ”–Μ”ΖΔ–‘±ψ”ΎΝάΗ…Θ®¥π“ΜΒψΨΆΕ‘Θ© Φλ≤ιΤχΟή–‘ ≤Μ‘Ό”–Τχ≈ί…ζ≥… ≈≈ΉΑ÷ΟΡΎΤχΧεΘ§ Ι…ζ≥…ΒΡΕΰ―θΜ·ΧΦΚΆΥ°’τΤχ±ΜΚσΟφΒΡΉΑ÷Ο≥δΖ÷Έϋ ’ CuCO3ΓΛCu(OH)2ΓΛ2H2O
ΓΨΫβΈωΓΩ
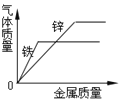
Θ®“ΜΘ©Θ®1Θ©ΓΑ‘χ«ύΒΟΧζ‘ρΜ·ΈΣΆ≠Γ± «Χζ”κΝρΥαΆ≠Ζ¥”Π…ζ≥…Ά≠ΚΆΝρΥα―«ΧζΘΜΙ ΧνΘΚFe+CuSO4=FeSO4+CuΘΜ
Θ®2Θ©Ά≠ΨΏ”–ΒΦΒγ–‘Θ§Ω…“‘÷Τ≥…ΒΦœΏΘΜΙ ΧνΘΚΒΦΒγ–‘ΘΜ
Θ®ΕΰΘ©Θ®1Θ©Κ§Ά≠ΖœΝœΖέΥιΒΡΡΩΒΡ «‘ω¥σΖ¥”ΠΈο÷°ΦδΒΡΫ”¥ΞΟφΜΐΘ§Φ”ΩλΖ¥”ΠΥΌ¬ ΘΜΙ ΧνΘΚ‘ω¥σΖ¥”ΠΈο÷°ΦδΒΡΫ”¥ΞΟφΜΐΘ§Φ”ΩλΖ¥”ΠΥΌ¬ ΘΜ
Θ®2Θ©H2O2 ή»»“ΉΖ÷ΫβΘ§Έ¬Ε»”ΠΗΟΩΊ÷Τ‘Ύ50ΓφΒΫ60Γφ÷°ΦδΘ§Ζά÷ΙΈ¬Ε»ΙΐΗΏΙΐ―θΜ·«βΖ÷ΫβΘΜΙ ΧνΘΚΖά÷ΙΈ¬Ε»ΙΐΗΏΙΐ―θΜ·«βΖ÷ΫβΘΜ
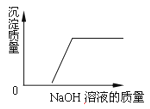
Θ®3Θ©–Υ»Λ–ΓΉιΆ§―ß“Μ÷¬÷Η≥ωΘ§ΫΪ¬Υ“ΚΨ≠’τΖΔ≈®ΥθΓΔά以ΫαΨßΓΔΙΐ¬ΥΒ»≤ΌΉςΒΟΒΫΝρΥαΆ≠ΨßΧεΘΜ”ΟΥ°œ¥Β”Ω…“‘»ήΫβΝρΥαΆ≠ΨßΧεΘ§ ΙΝρΥαΆ≠ΨßΧε”–Υπ ßΘ§Υυ“‘ΥυΒΟΝρΥαΆ≠ΨßΧε–η“Σœ¥Β”ΚσΝάΗ…Θ§ΉνΚœ ΒΡœ¥Β” ‘ΦΝ «95%ΒΡΨΤΨΪΘ§“ρΈΣΝρΥαΆ≠ΨßΧε≤Μ»ή”ΎΨΤΨΪΘ§«“ΨΤΨΪ”–Μ”ΖΔ–‘±ψ”ΎΝάΗ…ΘΜΙ ΧνΘΚά以ΫαΨßΘΜΖά÷ΙΨßΧε»ή”ΎΥ°¥χά¥ΥπΚΡΜρΨΤΨΪ”–Μ”ΖΔ–‘±ψ”ΎΝάΗ…ΘΜ
Θ®»ΐΘ©Θ®1Θ© Β―ι«Α”ΠΗΟœ»Φλ≤ιΤχΟή–‘Θ§‘ΌΙΡ»κ“ΜΕΈ ±ΦδΒΣΤχΚσ≥ΤΝΩBΓΔCΓΔDΓΔEΒΡ÷ ΝΩΓΘΙ ΧνΘΚΦλ≤ιΤχΟή–‘ΘΜ
Θ®2Θ©xCuCO3ΓΛyCu(OH)2ΓΛzH2OΦ”»»÷Ν200 ±ΜαΖ÷ΫβΒΟΒΫ―θΜ·Ά≠Θ§Εΰ―θΜ·ΧΦΚΆΥ°ΓΘΆΘ÷ΙΙΡ»κΒΣΤχΘ§ΒςΩΊΈ¬ΒγΦ”»»220Γφ≥÷–χΦ”»»Θ§Ιέ≤λΒΫΉΑ÷ΟB÷–≤Μ‘Ό”–Τχ≈ί…ζ≥… ±±μΟςΦν ΫΧΦΥαΆ≠“―Ψ≠Άξ»ΪΖ÷ΫβΓΘΙ ΧνΘΚ≤Μ‘Ό”–Τχ≈ί…ζ≥…ΘΜ
Θ®3Θ©Ζ¥”ΠΫα χΚσΘ§‘ΌΜΚΜΚΙΡ»κ“ΜΕΈ ±ΦδΒΣΤχΒΡΡΩΒΡ «≈≈ΉΑ÷ΟΡΎΤχΧεΘ§ Ι…ζ≥…ΒΡΕΰ―θΜ·ΧΦΚΆΥ°’τΤχ±ΜΚσΟφΒΡΉΑ÷Ο≥δΖ÷Έϋ ’ΓΘΙ ΧνΘΚ≈≈ΉΑ÷ΟΡΎΤχΧεΘ§ Ι…ζ≥…ΒΡΕΰ―θΜ·ΧΦΚΆΥ°’τΤχ±ΜΚσΟφΒΡΉΑ÷Ο≥δΖ÷Έϋ ’ΘΜ
Θ®4Θ©B÷–»ή“Κ÷ ΝΩ‘ωΦ”5.4gΘ§C÷–»ή“Κ÷ ΝΩ‘ωΦ”4.4gΘ§Υυ“‘…ζ≥…Υ°ΒΡ÷ ΝΩΈΣ5.4gΘ§Εΰ―θΜ·ΧΦΒΡ÷ ΝΩΈΣ4.2gΘ§A÷–…ζ≥…―θΜ·Ά≠ΙΧΧεΒΡ÷ ΝΩΈΣ25.8g-4.4g-5.4g=16gΘ§
…ηΧΦΥαΆ≠ΒΡ÷ ΝΩΈΣxΘ§…ζ≥…―θΜ·Ά≠ΒΡ÷ ΝΩΈΣy

x=12.4g y=8g
Υυ“‘«β―θΜ·Ά≠Φ”»»Ζ÷Ϋβ…ζ≥…―θΜ·Ά≠ΒΡ÷ ΝΩΈΣ16g-8g=8g
…η«β―θΜ·Ά≠ΒΡ÷ ΝΩΈΣzΘ§…ζ≥…Υ°ΒΡ÷ ΝΩΈΣa
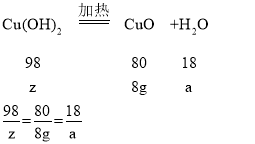
z=9.8g a=1.8g
Υυ“‘xCuCO3ΓΛyCu(OH)2ΓΛzH2O÷–ΫαΨßΥ°ΒΡ÷ ΝΩΈΣ5.4g-1.8g=3.6g
ΗυΨίΧβ“β”–ΘΚ![]() xΘΚyΘΚz
xΘΚyΘΚz
Ά®ΙΐΦΤΥψΩ…ΒΟΘΚxΘΚyΘΚz=1ΘΚ1ΘΚ2
Υυ“‘ΗΟΦν ΫΧΦΥαΆ≠ΒΡΜ·―ß Ϋ «CuCO3ΓΛCu(OH)2ΓΛ2H2OΓΘ
Ι ΧνΘΚCuCO3ΓΛCu(OH)2ΓΛ2H2OΓΘ

 ΟœΫ®ΤΫ¥μΧβ±ΨœΒΝ–¥πΑΗ
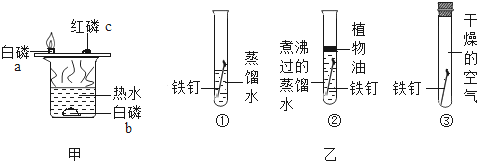
ΟœΫ®ΤΫ¥μΧβ±ΨœΒΝ–¥πΑΗ ≥§Ρή―ßΒδ”Π”ΟΧβΧβΩ®œΒΝ–¥πΑΗ
≥§Ρή―ßΒδ”Π”ΟΧβΧβΩ®œΒΝ–¥πΑΗΓΨΧβΡΩΓΩΫχ»κΕ§ΦΨΘ§Έμω≤ΧλΤχΤΒΖ±≥ωœ÷ΓΘΈμ «Ω’Τχ÷–Υ°’τΤχΡΐΫαΒΡΈΔ–ΓΥ°ΒΈ¥σΝΩ–ϋΗΓ‘ΎΫϋΒΊΟφΩ’Τχ÷––Έ ≥…ΒΡΘΜω≤–Έ≥…ΒΡ÷ς“Σ‘≠“ρ «ΙΛ“Β»ΦΟΚΓΔ≥”ϻΦΝœ≤Μ≥δΖ÷»Φ…’≤ζ…ζΒΡΧΩΝΘΓΔΧΦ«βΜ·ΚœΈο“‘ΦΑΫ®÷ΰ≥ΨΑΘ Β»¥σΝΩΜλ»κΩ’Τχ–Έ≥…ΒΡΓΘΕΰ’ΏΫαΚœ–Έ≥…Έμω≤ΓΘΜ·―ß–Υ»Λ–ΓΉιΒΡΆ§―ßΟ« ’Φ·ΝΥΩ’ΤχΨΜΜ·Τς¬Υ–Ψ…œΒΡ…Ό ΝΩΚΎ…ΪΈο÷ Θ§Ε‘Τδ≥…Ζ÷Ϋχ––ΝΥΧΫΨΩΓΘ
Θ®Χα≥ωΈ ΧβΘ©Ω’ΤχΨΜΜ·Τς¬Υ–Ψ…œΒΡΚΎ…ΪΈο÷ ÷– «ΖώΚ§”–ΧΩΝΘΚΆΥ°ΘΩ
Θ®≤ι‘ΡΉ ΝœΘ©
A ΧΦ«βΜ·ΚœΈοΨΏ”–Ω…»Φ–‘Θ§»Φ…’…ζ≥…Υ°ΚΆΕΰ―θΜ·ΧΦΓΘB ΈόΥ°ΝρΥαΆ≠ΈΣΑΉ…ΪΙΧΧεΘ§”ωΥ°Μα±δάΕΓΘ
Θ®Ϋχ–– Β―ιΘ©
Ά§―ßΟ«‘Ύάœ ΠΒΡ÷ΗΒΦœ¬Ε‘ΤδΫχ––ΧΫΨΩΘ§œ¬ΆΦΈΣΧΫΨΩΙΐ≥Χ÷–ΒΡ≤ΩΖ÷ΉΑ÷ΟΓΘΦ”»»«Αœ»œρΉΑ÷Ο A ÷–Ά®»κΉψΝΩ―θΤχΘ§≈≈ΨΓΩ’ΤχΓΘ’β―υΉωΒΡΡΩΒΡ «ΔΌ_________ΓΘ

Β―ι≤ΌΉς | Β―ιœ÷œσ | Β―ιΫα¬έ |
Βψ»ΦΨΤΨΪΒΤΘ§≥δΖ÷Ζ¥”Π | ΔΎ________ | ΚΎ…ΪΈο÷ ÷–Κ§”–ΧΩΝΘΚΆΥ° |
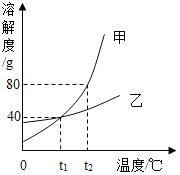
Θ®Ζ¥ΥΦ”κΤάΦέΘ©’≈Ά§―ß»œΈΣΗΟΫα¬έ≤Μ―œΫςΘ§ΥϊΒΡάμ”… «Δέ_________ΓΘ
Θ®ΫΜΝς”κΧ÷¬έΘ©Ν§Ϋ”ΉΑ÷ΟΒΡ’ΐ»ΖΥ≥–ρ»γ…œΆΦΥυ ΨΓΘ»τΫΪΉΑ÷Ο BΓΔC ΒΡΈΜ÷ΟΕ‘ΒςΘ§ΜαΕ‘ Β―ιΫαΙϊ≤ζ…ζΒΡ”Αœλ «Δή_________ΓΘ
Θ®ΆΊ’Ι―”…λΘ©Έμω≤ΒΡ≥…Ζ÷Ζ«≥ΘΗ¥‘”Θ§Ά®Ιΐ“‘…œ Β―ι÷ΜΡή÷ΛΟςΚΎ…ΪΈο÷ ÷–Κ§”–ΧΦ‘ΣΥΊΚΆ«β‘ΣΥΊΘ§»τ“ΣΝΥΫβΈμω≤ΒΡ≥…Ζ÷Θ§–ηΫχ“Μ≤ΫΒΡ―ßœΑΚΆ―–ΨΩΓΘΆ§―ßΟ«‘Ύ Άχ…œ≤ι‘ΡΉ ΝœΜΙΖΔœ÷ΝΥ“Μ÷÷ΖάΈμω≤ΩΎ’÷Θ®»γΆΦΘ©Θ§“―÷ΣΡ≥ΤΖ≈Τ KN95 –ΆΖάΩ≈ΝΘΈοΩΎ’÷ Ι”ΟΝΥΈόΖΡ≤ΦΓΔΜν–‘ΧΩΒ»≤ΡΝœΘ§Τδ÷–ΈόΖΡ≤ΦΚΆΜν–‘ΧΩΕ‘Ω≈ΝΘΈο ΚΆ”–ΚΠΤχΧεΡήΖ÷±πΤπΒΫΔί_________Ής”ΟΓΘ