��Ŀ����
����Ŀ����1��С���ȼ�λͬѧ�����м������ʯ��ˮ������������Һ��ʵ��̽����������룺
��������⣩��μ�����������ɫ��Һ��
��ʵ�鷽�������ǽ�������ͼ��ʾ��ʵ��

����ش��������⣺
������ʵ���в��ܴﵽʵ��Ŀ����___________������ĸ����
��C��ʵ���з�Ӧ�Ļ�ѧ����ʽΪ________________________��
��D��ʵ���б���ǵ�ԭ��Һ��_______________��
������̽����ʵ�������С��ͬѧ��A��B��C��D�����Թ��е�����ȫ������ͬһ���ɾ����ձ��У���ַ�Ӧ�õ���ɫ����������Һ���Ը���Һ�ijɷ��ֽ�����̽����
��������⣩����Һ�г�ˮ����̪�������Щ���ʣ�
���������ϣ��Ȼ�����Һ�����ԣ�
����������裩
��_______________
��NaCl��CaCl2��HCl
��NaCl��CaCl2��NaOH
����˼����չ�������������������ֻ��һ��������������_____������ţ���������________��
�ڸ�����ѧ��ѧ֪ʶ����֤�ձ�����Һ�п����е������Ƿ���ڣ�������Щ���ʵ���ʹ�ò�����ɸ�ʵ��___________������ĸ��
a pH��ֽ b ��������Һ c ��ɫʯ����Һ d ͭ e ��������������Һ��
��2����������������������������Ҫ��Դ��
��������⣩�����ǵ�ȼ�ղ�����CO2��H2O���ɴ��ܷ�֤����������ֻ��̼Ԫ�غ���Ԫ����ɵ��л��
�����ʵ�飩Ϊ��ȷ�������ǵ�Ԫ����ɣ�ijС�����������ʵ�飨����Ũ���ᡢ��ˮCaCl2��Ϊ���ø���������̶ֹ�װ��ʡ�ԣ���
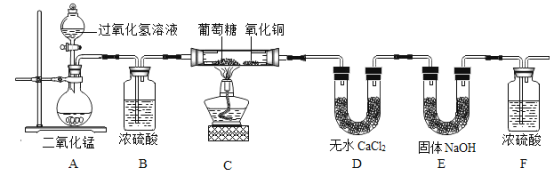
��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��__________________________��
��װ��B��Ũ�����������_________________________��
��װ��C������ͭ��������_________________________��
���������ۣ�
���ó��п����Ĵ��������װ��A���Ƿ��������ʵ��Ľ��У�_____��������������������ԭ����______________________��
��װ��C��������ȼ�յ�����ص���_________________������һ������
�����ݴ������±���ͬѧ����д��ʵ�鱨�棬���������ɡ�
ʵ����ʵ | ���ݷ��������� |
1.8g��������ȫȼ�գ��õ�2.64gCO2��1.08gH2O | ����___________________________ ���ۣ������Ǻ���C��H��O����Ԫ�� |
�����۽�����Ϊ�˾���������
��1����ʵ���ڽ��й�����Ӧע���������_______________��1������
��2���Ӷ���ʵ��ĽǶȿ�����ʵ��ɽ�һ���Ľ������Ҫд��һ���Ľ������________��
���𰸡�A��B Na2CO3+Ca��OH��2=CaCO3��+2NaOH ����ʯ��ˮ����Ca��OH��2��Һ�� NaCl��CaCl2 �� NaOH��Һ��ʹ��ɫ��̪��Һ��죬���ܵõ���ɫ��Һ b��d 2H2O2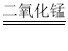 2H2O +O2�� �������� �������Dz���ȫ����������COת��ΪCO2����֤�������е�̼��ת��ΪCO2 �� �����еĶ�����̼�����ʵ�����IJⶨ������������������ͣ�������������Ǵ�������ȫȼ�գ�Ӱ�����ⶨ�� ���ܱ���ϵ�н���ʵ�飬�����ȷ 2.64g������̼�к���̼Ԫ��0.72g��1.08gˮ�к�����Ԫ��0.12g̼��Ԫ�ص�����֮��Ϊ0.84gС��1.8g�����ǵ����������������Ǻ���C��H��O����Ԫ�أ�����������ֻ����C��H����Ԫ�أ�����O2��2.64g+1.08g-1.8g=1.92g����2.64gCO2����������Ϊ1.92g��1.08gH2O����������Ϊ0.96g����Ϊ�����й�������1.92g+0.96g=2.88g>1.92g�����������Ǻ���C��H��O����Ԫ�أ� ����A�в������������� ����������������ֹȼ�ղ���֣������װ����ȴ���ٳ���D��E��������������ͨ�����ٵ�ȼC���ƾ��ƣ� ����β������װ�ã���F����װ��NaOH�ĸ���ܣ�ͬʱ���տ����е�CO2��ˮ������ʵ����������A��B���һ���ռ���ƿ���õ������ӣ�
2H2O +O2�� �������� �������Dz���ȫ����������COת��ΪCO2����֤�������е�̼��ת��ΪCO2 �� �����еĶ�����̼�����ʵ�����IJⶨ������������������ͣ�������������Ǵ�������ȫȼ�գ�Ӱ�����ⶨ�� ���ܱ���ϵ�н���ʵ�飬�����ȷ 2.64g������̼�к���̼Ԫ��0.72g��1.08gˮ�к�����Ԫ��0.12g̼��Ԫ�ص�����֮��Ϊ0.84gС��1.8g�����ǵ����������������Ǻ���C��H��O����Ԫ�أ�����������ֻ����C��H����Ԫ�أ�����O2��2.64g+1.08g-1.8g=1.92g����2.64gCO2����������Ϊ1.92g��1.08gH2O����������Ϊ0.96g����Ϊ�����й�������1.92g+0.96g=2.88g>1.92g�����������Ǻ���C��H��O����Ԫ�أ� ����A�в������������� ����������������ֹȼ�ղ���֣������װ����ȴ���ٳ���D��E��������������ͨ�����ٵ�ȼC���ƾ��ƣ� ����β������װ�ã���F����װ��NaOH�ĸ���ܣ�ͬʱ���տ����е�CO2��ˮ������ʵ����������A��B���һ���ռ���ƿ���õ������ӣ�
��������
��1��{ʵ�鷽��}��A ���������ʯ��ˮ���������ƾ��ܷ����кͷ�Ӧ�������������Եı仯���������ֳ���ʯ��ˮ������������Һ��
B ����ʯ��ˮ������������Һ���ʼ��ԣ�����ʹ��̪��Һ��죬���÷�̪�������ֳ���ʯ��ˮ������������Һ��
C ̼������Һ�����ʯ��ˮ��Ӧ������ɫ������������������Һ��������Ӧ����̼������Һ�������ֳ���ʯ��ˮ������������Һ��
D ������̼���������ʯ��ˮ��Ӧ������ɫ���������������Ʒ�Ӧ���������Ա仯�����ö�����̼�������ֳ���ʯ��ˮ������������Һ��������ʵ���в��ܴﵽʵ��Ŀ����A��B������AB��
��C��ʵ���еķ�Ӧ��̼���������ʯ��ˮ�е��������Ʒ�Ӧ����̼��Ƴ������������ƣ��ʷ�Ӧ�Ļ�ѧ����ʽдΪ��Na2CO3+Ca(OH)2=CaCO3��+2NaOH��
�۶�����̼�������ʯ��ˮ�е��������Ʒ�Ӧ����̼��Ƴ���ʹҺ�����ǣ�����D��ʵ���б���ǵ�ԭ��Һ�dz����ʯ��ˮ���������ʯ��ˮ����Ca(OH)2��Һ����
{���������}��Ӧ�õ���ɫ����������Һ��˵����Һ�в�������ʹ��̪�����������ƺ�̼���ƣ�������Һ�е��������Ȼ��ơ��Ȼ��ƣ�����NaCl��CaCl2��
{��˼����չ}�����������������ֻ��һ�������������Ǣ�NaCl��CaCl2��NaOH������III��
����������Һ�ʼ��ԣ���ʹ��Һ�еķ�̪��죬���ܵõ���ɫ��Һ������NaOH��Һ��ʹ��ɫ��̪��Һ��죬���ܵõ���ɫ��Һ��
��a ͨ��pH��ֽ����Һ��pH�����ж���Һ������ԣ��ܹ��ж���Һ�еĿ��ܴ������ʵ������ѡ����ȷ��
b ��������Һ�����Ȼ��ơ��Ȼ��Ʒ�Ӧ������ɫ�����Ȼ�����Ҳ�������ᷴӦ���ɰ�ɫ�����Ȼ����������жϿ��ܴ������ʵĴ��������ѡ�����
c ��ɫʯ����Һͨ����ɫ�ı仯ָʾ��Һ������ԣ��ܹ��ж���Һ�еĿ��ܴ������ʵ������ѡ����ȷ��
d ͭ����Һ�е�һ�����ڵ����ʺͿ��ܴ��ڵ����ʾ���������Ӧ�������жϿ��ܴ������ʵĴ��������ѡ�����
e ����������������Һ�ӵ���Һ�У������������죬˵�����ܴ��ڵ�����-----������ڣ����������죬˵��������ڣ�ѡ����ȷ������b��d��
��2��{���ʵ��}����ͼ��֪��װ��A�з����ķ�Ӧ�ǹ��������ڶ��������������������·�Ӧ����ˮ���������ʷ�Ӧ�Ļ�ѧ����ʽдΪ��2H2O2 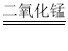 2H2O +O2����
2H2O +O2����
��Ũ���������ˮ�ԣ�����װ��B��Ũ����������Ǹ����������������������
������ͭ���������ԣ�װ��C������ͭ�������ǽ������Dz���ȫ����������COת��ΪCO2����֤�������е�̼��ת��ΪCO2����������Dz���ȫ����������COת��ΪCO2����֤�������е�̼��ת��ΪCO2��
{��������} �ٿ������ж��ֳɷ֣����ʵ��������ţ������
�����еĶ�����̼�����ʵ�����IJⶨ������˵���������������ͣ�������������Ǵ�������ȫȼ�գ�Ӱ�����ⶨ����������еĶ�����̼�����ʵ�����IJⶨ������������������ͣ�������������Ǵ�������ȫȼ�գ�Ӱ�����ⶨ����
��װ��C�����������ܱ�װ����ȼ�գ������˿�������������еijɷֶ�ʵ��������ţ��������ܱ���ϵ�н���ʵ�飬�����ȷ��
{���ݴ���} ���ɵ�2.64g������̼�к���̼Ԫ�ص�����Ϊ 2.64g��![]() ��100%=0.72g�����ɵ�1.08gˮ�к�����Ԫ�ص�����Ϊ1.08g��
��100%=0.72g�����ɵ�1.08gˮ�к�����Ԫ�ص�����Ϊ1.08g��![]() =0.12g�����������غ㶨�ɣ�1.8g��������̼��Ԫ�ص�����֮��Ϊ0.72g+0.12g=0.84g��0.84gС��1.8g�����ǵ����������������Ǻ���C��H��O����Ԫ�أ�����2.64g������̼�к���̼Ԫ��0.72g��1.08gˮ�к�����Ԫ��0.12g̼��Ԫ�ص�����֮��Ϊ0.84gС��1.8g�����ǵ����������������Ǻ���C��H��O����Ԫ�ء�
=0.12g�����������غ㶨�ɣ�1.8g��������̼��Ԫ�ص�����֮��Ϊ0.72g+0.12g=0.84g��0.84gС��1.8g�����ǵ����������������Ǻ���C��H��O����Ԫ�أ�����2.64g������̼�к���̼Ԫ��0.72g��1.08gˮ�к�����Ԫ��0.12g̼��Ԫ�ص�����֮��Ϊ0.84gС��1.8g�����ǵ����������������Ǻ���C��H��O����Ԫ�ء�
{���۽���}�ٸ�ʵ���ڽ��й�����Ӧע��������У�1������A�в������������� ����������������ֹȼ�ղ���֣���2����װ����ȴ���ٳ���D��E��������3����ͨ�����ٵ�ȼC���ƾ��ƣ��������A�в������������� ����������������ֹȼ�ղ���֣������װ����ȴ���ٳ���
��Ϊ����E���������ƹ������տ����еĶ�����̼��ʵ��������ţ��ɽ�F����װ��NaOH�ĸ���ܣ�ͬʱ���տ����е�CO2��ˮ������ʵ�������F����װ��NaOH�ĸ���ܣ�ͬʱ���տ����е�CO2��ˮ������ʵ����