��Ŀ����
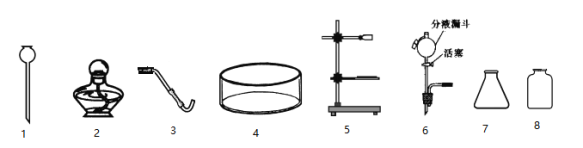
����Ŀ������ͼ��ʾΪʵ�����г��������Ʊ�������ռ�������ʵ��IJ�����������װʵ��װ��ʱ�����ظ�ѡ��λ�������Ը�����ĿҪ�ش��������⣺
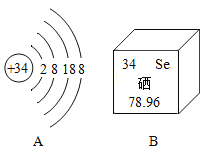
��1��������أ�KMnO4�������ڼ��������£��ܽϿ�طֽ�Ϊ����أ�K2MnO4�����ܹ��壩���������̣����ܹ��壩�����������Ը������Ϊԭ����ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ___________����д���������ĸ��
���ø�������Ʊ�����������Ӧ�Ļ�ѧ����ʽΪ_______________________________��
�����Ӹ��������ȫ��Ӧ��Ĺ��������л��ն������̣���Ҫ�IJ�������Ϊ���ܽ���___________��ϴ����������õ������Ķ������̹��塣
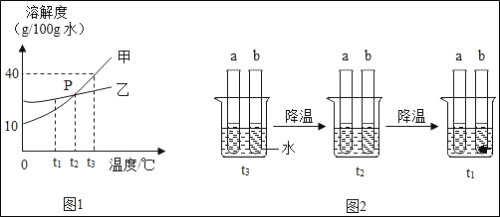
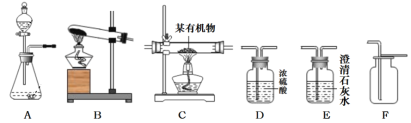
��2�����Թ���������ҺΪԭ�ϣ���ʵ��������ȡ����������ij�л�������Ԫ�ؽ��з���̽����������ʾ���л�����һ����C��HԪ�أ����ܺ�OԪ�أ�����ѡ����������˳��Ϊ��AһD1��CһD2��E����ʯ�Ҹ���ܣ���֪��D1��D2Ϊ2��Ũ����ϴ��ƿ����ʹ���л����ڴ����г��ȼ�գ��۲������ռ��й�ʵ�������ݣ�����������з����ķ�Ӧ��ǡ����ȫ���У���
���ڸ�װ���У�����D1��D2�����ö�������ˮ�֣������õIJ�֮ͬ���ǣ�________��
������E�۲쵽��������_______________��
��Ӧ��ɺ�����E����Һ�������ȷ�Ӧǰ��Һ������__________������ţ���
A��� B����С C������ D����ȷ��
������C�Ĵ������з�����л���2.3g�����ȼ�պ������D2����������2.7g������E����4.4g������л�����̼����ԭ�ӵĸ�����___________������������ȣ������л������Ƿ�����Ԫ��____________��������������������ȷ����֮һ����
���𰸡�B��D��F ![]() ���� ���� D1Ϊ���ų�ˮ�ֵĸ��ţ�����D2��Ϊ�����ݲɼ����ж������� ����ʯ��ˮ����ǣ� B 1�� 3�� ��
���� ���� D1Ϊ���ų�ˮ�ֵĸ��ţ�����D2��Ϊ�����ݲɼ����ж������� ����ʯ��ˮ����ǣ� B 1�� 3�� ��
��������
��1���ٸ��������ȡ����Ϊ������ȷ�Ӧ�����ɵ�������Ũ�������������ſ������ռ�������������˳��Ϊ��B��D��F��
�ڸ���������ȷֽ���������ء��������̡���������ѧ����ʽΪ��![]() ��
��
�۶������̲�����ˮ���ܽ��ͨ�����˲����ɵõ��������̣�
��2��������D1��Ϊ���ų������л���ˮ�ֵĸ��ţ�����D2��Ϊ�˲ⶨ�л���ȼ�պ�����ˮ��������
��E���Լ�Ϊ����ʯ��ˮ���л��ﺬ̼Ԫ�أ�ȼ�����ɶ�����̼����ʹ����ʯ��ˮ����ǣ�E�г���ʯ��ˮÿ����44�������Ķ�����̼������100��������̼��Ƴ�������Һ������������Һ������С��
�۸��л�����̼Ԫ������Ϊ��![]() ����Ԫ������Ϊ
����Ԫ������Ϊ![]() ����̼����ԭ�Ӹ�����Ϊ��
����̼����ԭ�Ӹ�����Ϊ��![]() ����Ϊ̼����Ԫ������֮�ͣ�1.2g+0.3g=1.5g��С�ڸ��л����������2.3g��,�ʸ��л���������Ԫ�ء�
����Ϊ̼����Ԫ������֮�ͣ�1.2g+0.3g=1.5g��С�ڸ��л����������2.3g��,�ʸ��л���������Ԫ�ء�

����Ŀ���ҹ����������պ��졢�ֻ�����ȶ��Ѿ���������һ��ˮƽ���������в��ϵ�Ӧ�ûش����⡣
Ӧ�� | �����˺��� �������� | ��������ˮ½���ܷɻ��㲿�� | ��Ϊ�۵��ֻ���Ļ |
�õ��IJ��� | ���ٸ� | �ѺϽ����Ͻ� | �����ǰ����� |
��1�����������������л��ϳɲ��ϵ���_________�����ڽ������ϵ���_________��дһ�ּ��ɣ���
��2���ɻ�������ѺϽ����Ͻ���ŵ���_________��
��3���ڶԸ����ֹ��еķ�϶���к���ʱ�����������������ڸ��������·�Ӧ��������״̬�µ�������һ��������÷�Ӧ�Ļ�ѧ����ʽΪ________����Ӧ����������________��