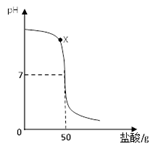
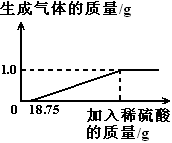
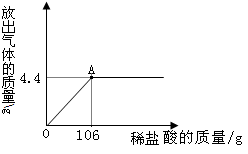
��Ŀ����
����ʹ�õ�ˮ���ײ�����һ��ˮ��������Ҫ�ɷ���̼��ƺ�������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±�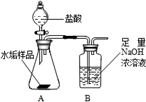
| | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| Bװ�����ӵ����� | 2.17 | 2.22 | 2.21 | |
��1����һ��ʵ�������ݽϵ͵�ԭ����___________��
��2��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ___________�ˣ�
2.20��5.00��
�������������ƽ��ֵ=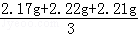 =2.20g��
=2.20g��
��1����һ��ʵ�����ɵĶ�����̼û��ȫ������Bװ����ȥ�����Ե�һ��ʵ�������ݽϵͣ������һ��ʵ�����ɵĶ�����̼û��ȫ������Bװ����ȥ��
��2����ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 2.20g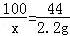
x=5.00g
��ƽ��ÿ��ˮ����Ʒ��̼��Ƶ�����Ϊ5.0g��
���㣺�йػ�ѧ����ʽ�ļ���

Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʡ���ԭ���ǰѵ������еĵ�Ԫ����ȫת���ɰ���(��ѧʽΪNH3)������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽ��2NH3+H2SO4=(NH4)2SO4
��ȡ��ţ����Ʒ30 mL���á��Ƕ�����ֽ����еĵ����ʣ������İ�����9.5 g������������Ϊ4.9%��ϡ����ǡ����ȫ���ա����㲢�ش��������⣺
(1)���������������Ƕ��ٿˣ�(��������ȷ��0.01 g����ͬ)
(2)30 mLţ���к���Ԫ�ص������Ƕ��ٿˣ�
(3)��ͼ�Ǹ�ţ�̰�װ��ǩ�IJ������ݡ���֪ţ���еĵ����ʺ���Ԫ�ص���������Ϊ16%������ͨ������ȷ������ţ����Ʒ�е����ʵĺ����Ƿ�ﵽ�˰�װ��ǩ����ʾ�ĵ����ʵ���������
| ���ϣ���ţ�� �����ڣ�8���� ��������250 mL/�� Ӫ���ɷ֣�(ÿ100 mL) �ơ�0.11 g ֬����3.30 g �����ʡ�2.90 g |