��Ŀ����
���ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õļ�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3������25.0g���������Ƴ���Һ������������μ���������������������Ϊ7.3%��ϡ���ᣬʹ������ȫ�ų������ռ���8.8g������̼���壮�Լ��㣺��1��ԭ������Na2CO3����������������2����Ӧ�����ĵ������������
��1��84.8%����2��200g��
���������������1����̼���Ƶ�����Ϊx��������Һ�����ʵ�����Ϊy��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 73 44
x y 8.8g
x=21.2g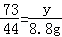
y=14.6g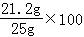 %=84.8%
%=84.8%
��ԭ������̼���Ƶ���������Ϊ84.8%��
��2����Ӧ�����ĵ������������Ϊ�� ="200g" �𣺷�Ӧ�����������������Ϊ200g��
="200g" �𣺷�Ӧ�����������������Ϊ200g��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ��㣻�й��������������ļ��㣮

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�ij�����������Ĵ����Ʒ�����������Ȼ��ƣ��İ�װ����ע����̼���ơ�96%��
Ϊ�˲ⶨ�ò�Ʒ��̼���Ƶ����������Ƿ���ʵ����4���ֱ�ʢ��Ũ����ͬ��������Ϊ100gϡ������ձ��У��ֱ���벻ͬ�����Ĵ����Ʒ����ַ�Ӧ�����ձ���ʣ�����������ʵ���������±���
| ������������ | ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
| ���봿���Ʒ����/g | 2.8 | 5.6 | 11.2 | 14 |
| �ձ���ʣ��������/g | 101.7 | 103.4 | 106.8 | 109.6 |
����㣨���������һλС������
��1���ô����Ʒ��̼�������������ı�ע�Ƿ���ʵ��
��2������������Һ�����ʵ�����������
��3��ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
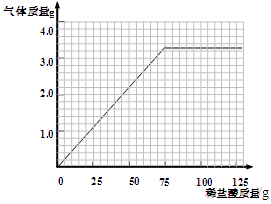
ijͬѧΪ�ⶨij����ʯ��̼��ƣ����裺����ʯ�е����ʲ������ᷴӦ��������������ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼����:
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 10.0 | 10.0 | 10.0 | 10.0 |
| ȡϡ����������g�� | 25.0 | 50.0 | 75.0 | 100.0 |
| ��������������g�� | 1.1 | X | 3.3 | 3.3 |
����㣺
��1�������������ڵ�1�β�õ������У� �������ʣ���ȫ��Ӧ�ˣ�
��2��������X= g��
��3���ô���ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
��4��������10.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
 KCl + 3O2�����ֽ� 29.4g ������� 5.6g �������̵Ļ�������һ��ʱ�����ȴ������ʣ����������Ϊ 25.4g���ش�
KCl + 3O2�����ֽ� 29.4g ������� 5.6g �������̵Ļ�������һ��ʱ�����ȴ������ʣ����������Ϊ 25.4g���ش�