��Ŀ����
����Ŀ����¯���������ԭ��������ʯ����̿��ʯ��ʯ�ȣ�����̿�����ͼ��Ҫ��ʾ��
��֪����١�����ں���۾����������
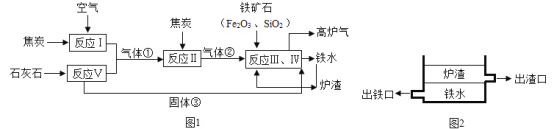
��1������ڵĻ�ѧʽΪ_____������������������Ʋ�¯������Ҫ�ɷ�Ϊ_____���ѧʽ����
��2��������¯�ײ�ͨ����ͬ�߶ȵij��ڷ�����ˮ��¯������ͼ2��ʾ������ʵ�����������ԭ��������Ϊ¯������_____��_____�����ʡ�
��3����Ӧ�������ڻ��Ϸ�Ӧ����_____�������ڷֽⷴӦ����_____���������û���Ӧ����_____����
��4����¯�����к��ж��ֳɷ֣����к�����ߣ�ռ55%��60%������_____���ѧʽ�����������25��30%�Ŀ�ȼ������_____���ѧʽ�����Լ�9��12%���ҵ�_____���ѧʽ����
���𰸡�CO CaSiO3 �۵�� �ܶȱ���ˮС 3 1 0 N2 CO CO2
��������
��1��̼�������ڵ�ȼ�����������ɶ�����̼��������̼��̼�ڸ��µ�����������һ����̼����������ڵĻ�ѧʽΪCO����ѧ��Ӧǰ��Ԫ������䣬��������������������Ʋ�¯������Ҫ�ɷ�Ϊ��CaSiO3��
��2��¯���ij������Ϸ�����ˮ�ij������·�������¯�������۵�ߡ��ܶȱ���ˮС�����ʣ�
��3������̼��������Ӧ���ɶ�����̼�����ڻ��Ϸ�Ӧ�����Ƕ�����̼��̼��������һ����̼�����ڻ��Ϸ�Ӧ������һ����̼���������ڸ��µ��������������Ͷ�����̼�������ڻ�����Ӧ���ͣ����������ơ��������跴Ӧ���ɹ���ƣ����ڻ��Ϸ�Ӧ������̼��Ƹ������������ƺͶ�����̼�����ڷֽⷴӦ�����Է�Ӧ�������ڻ��Ϸ�Ӧ����3�������ڷֽⷴӦ����1���������û���Ӧ����0����
��4�������к�����ߵ��ǵ��������Ը�¯���к��ж��ֳɷ֣����к�����ߣ�ռ55��60%������N2���������25��30%�Ŀ�ȼ������CO���Լ�9��12%���ҵ�CO2��

����Ŀ���ܱ���������������X��ˮ�Ͷ�����̼4�����ʣ���һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ��������±���ʾ������˵����ȷ���ǣ� ��
���� |
| X |
|
|
��Ӧǰ����/g | 76.0 | 16.0 | 4.0 | 3.0 |
��Ӧ������/g | ���� | 0 | 40.0 | 47.0 |
A.���д����ֵΪ5.0
B.X��̼���⡢������Ԫ�����
C.�÷�Ӧ��![]() ��
��![]() �Ļ�ѧ������֮��Ϊ2:1
�Ļ�ѧ������֮��Ϊ2:1
D.��Ӧ���ɵ�![]() ��
��![]() ��������Ϊ40:47
��������Ϊ40:47