��Ŀ����
����Ŀ�����������������Ӧ�ù㷺��
��1�����۽Ƕȷ�����ˮ���¶ȼƲ������µ�ԭ����____________________________��
��2��д����ҵ���ó�����ʯ�����Ļ�ѧ����ʽ____________________________________��
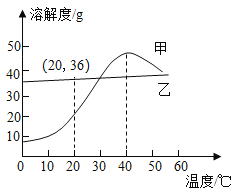
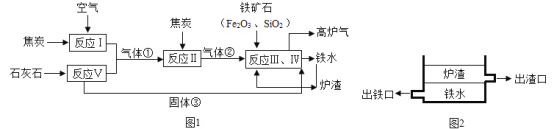
��3����ͼ�ǹ���������һϵ�б仯��
![]()
����������ʴ��Ҫ����������е�____________������ѧ��Ӧ��
����ϡ���������Ļ�ѧ����ʽΪ____________________________��
�������õ���ҺX�еĽ���������Ϊ _________���÷��ű�ʾ����
��4��Ϊ��֤Fe��Cu��Ag���ֽ����Ļ��˳������ѡ�õ�ҩƷ���е���____������ĸ����
A Fe��CuSO4��Һ��Ag B FeSO4��Һ��Cu��Ag
C FeSO4��Һ��Cu��AgNO3��ҺD FeSO4��Һ��CuSO4��Һ��Ag
��5��ij������ĩ����������þ��п���������е�һ�ֻ�����ɡ�ȡ����Ʒ2.4g�������м���100gһ��������������ϡ���ᣬǡ����ȫ��Ӧ������0.2g�������õ���ɫ��Һ��������ʣ�ࡣ����˵����ȷ����__________������ĸ����
A ��Ʒ��һ������п B ��Ʒ��һ���������������ܺ�����
C ������Һ������������Ϊ12g D �����ϡ�����������������Ϊ9.8%
���𰸡��¶����߹�ԭ��֮��ļ�϶��� Fe2O3+ 3CO![]() 2Fe + 3CO2 ������ˮ 6HCl +Fe2O3=2FeCl3+3H2O Cu2+ AC CD
2Fe + 3CO2 ������ˮ 6HCl +Fe2O3=2FeCl3+3H2O Cu2+ AC CD
��������
��1��ˮ���¶ȼ����ڲ������£���Ҫ��������Һ���������������ʣ��䱾�����¶����߹�ԭ��֮��ļ�϶���
��2�����������£���������һ����̼��Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe2O3+ 3CO![]() 2Fe + 3CO2��
2Fe + 3CO2��
��3������������ʴ��Ҫ������е�������ˮ������ѧ��Ӧ��
���������Ҫ�ɷ�����������ϡ��������������Ӧ�����Ȼ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ6HCl +Fe2O3=2FeCl3+3H2O��
����������ͭ��Һ��ͭ������Һ��Ӧ������ͭ�������������ʢ������õ���ҺX�еĽ���������Ϊͭ���ӣ�����ΪCu2+��
��4�����Ƚ�Fe��Cu��Ag���ֽ������������˳�������ã�Ȼ��ȡ�м�λ�õĽ�������������ȡ�����Ŀ���������Һ��ȡ�м�λ�õĽ����Ŀ���������Һ����������ȡ��������ѡAC��
��5��A������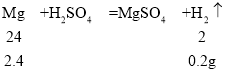 ��֪������0.2g������2.4gþ��ͬ���ɴ˿�֪������0.2g������������п�����������ֱ�Ϊ6.5g��1.8g��������Ʒ������þ��Ҳ����������п�Ļ�������
��֪������0.2g������2.4gþ��ͬ���ɴ˿�֪������0.2g������������п�����������ֱ�Ϊ6.5g��1.8g��������Ʒ������þ��Ҳ����������п�Ļ�������
B������Ʒ����100gһ��������������ϡ���ᣬǡ����ȫ��Ӧ������0.2g�������õ���ɫ��Һ��������ʣ�ࡣ˵����Ʒһ������������������
C���������֪�����������������ȫ��������������Σ���Һ�����������ε�����Ӧ���ڽ�����������������ӵ������ͣ�����������Ϊ2.4g����������ӵ�����=0.2g��![]() -0.2g=9.6g���������������ε�����=2.4g+9.6g=12g����ȷ��
-0.2g=9.6g���������������ε�����=2.4g+9.6g=12g����ȷ��
D�����������=0.2g��![]() =9.8g�������ϡ�����������������=
=9.8g�������ϡ�����������������=![]() ��100%=9.8%����ȷ����ѡCD��
��100%=9.8%����ȷ����ѡCD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�