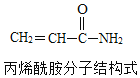
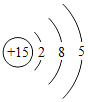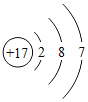��Ŀ����
����Ŀ������������ѧϰ��ѧ���õķ�����
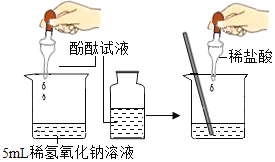
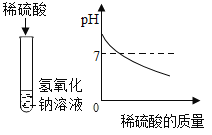
(1)����ʵ�鶼��̽�������֮���ܷ�����Ӧ����ӡ������о��������о����ĽǶȣ�������ʵ���е�A��_________(����ĸ)��Ϊһ�࣬������_____________��
|
��������������ͭ�����м���������ϡ���� |
������������Һ���ϵμ�ϡ���ᣬ�ӱ�����ȼƲⶨ��Һ��pH |
A | B | C |
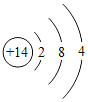
(2)�ǽ�����������Ӧ������Խ���ס���ӦԽ���ң���ǽ���Ԫ�صķǽ�����Խǿ���ݴ˹��ɣ����ƶϳ��������ڷǽ���Ԫ�صķǽ�����ǿ����˳��Ϊ__________________;�ṹ�������ʣ������λ�ڵ�������Ԫ�ص�ԭ�ӽṹ��Ԫ�����ʵĵݱ����______________________��
Ԫ�� | Si | p | S | Cl |
�����������ķ�Ӧ���� | ���� | �������������ܷ�Ӧ | ���� | ���Ȼ��ȼʱ������ը |
ԭ�Ӻ�������Ų� |
|
|
|
|
���𰸡�B AB���Ƕ����о� Cl��S��P��Si ����������Խ�࣬�ǽ�����Խǿ
��������
��1��AB���Ƕ����о�����C�Ƕ����о�����������ʵ���е�A��B��Ϊһ�࣬������AB���Ƕ����о���
���B��AB���Ƕ����о���
��2���������ڷǽ���Ԫ���У��������ҵĹ�Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ����������Ӧ������Խ��Խ���ף���˷ǽ�����ǿ����˳��Ϊ��������������ǿ��
�������ڷǽ���Ԫ���У�������������������Խ�࣬�ǽ�����Խǿ����˵�������Ԫ�ص�ԭ�ӽṹ��Ԫ�����ʵĵݱ����������������Խ�࣬�ǽ�����Խǿ��
���Cl��S��P��Si������������Խ�࣬�ǽ�����Խǿ��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�����Ŀ��С��ͬѧΪ��̽��ij������Ĵ���,�Ⱥ����������ʵ�飬ʵ���������±�
ʵ����� | ��һ�� | �ڶ��� | ������ |
��ȡ����ʯ��Ʒ������/g | 20 | 20 | 40 |
����ϡ���������/g | 500 | 400 | 300 |
�ձ���ʣ����������/g | 4 | 4 | 24 |
��������ͬѧ��ʵ��,�Իش���������: (������ʯ��Ʒ���������ʲ��������κ����ʷ�ӦҲ������ˮ)
(1)ʵ���з�����Ӧ�Ļ�ѧ����ʽΪ___________��
(2)�˳�����ʯ��Ʒ�Ĵ���Ϊ____________��
(3)������֪�����г���������ʵ���вμӷ�Ӧ����������(x) �ı���ʽ____________��
(4)�����98%��Ũ�������Ƶڶ���ʵ������ϡ���ᣬ���ˮ������Ϊ__________________��
(5)��������ʵ����ձ������ʹ��ˣ������ò�������Һ�м���184gˮ����������Һ��������������Ϊ__________________��
(6)�����������ʵ���е�ʣ����徭ϴ�ӡ��������������һ����̼���л�ԭ�������տɵõ����������Ϊ__________________��
����Ŀ������ˮ���ҹ�Ŀǰ��Ҫ����������ˮ���±����ҹ��䲼����������ˮˮ�ʱ��IJ������ݡ�
��Ŀ | �� |
�й�ָ�� | ����ζ������� |
��ѧָ�� | pH6.5-8.5�� ͭ<1.0mg. L-1����<0.3mg. L-1�������ȡ�0.3mg. L-1�� |
��1���й�ָ����ֵ�������ˮ��____________���ʣ�����������������ѧ����������ѧָ���е�pH��8ʱ����������ˮ��_____________������������������������������������
��2������ˮ�����õ��������ж������ȣ�CIO2 ����Ư��[Ca(CIO)2 ]���� ����Һ���� NaClO���ȡ���ҵ����ȡƯ�۵Ļ�ѧ����ʽΪ![]() ����ȡ��84����Һ�� �ǽ�����ͨ���ռ���Һ�У���Ӧԭ����Ư�۵���ȡ���ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ��______________��
����ȡ��84����Һ�� �ǽ�����ͨ���ռ���Һ�У���Ӧԭ����Ư�۵���ȡ���ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ��______________��
��3�������жԾ�ˮͨ��____________�ķ����ȿ�������ɱ����Ҳ���Խ���ˮ��Ӳ�ȡ�