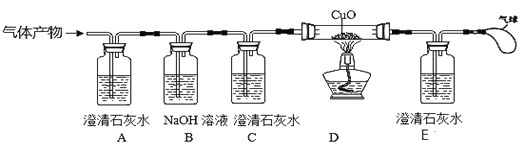
��Ŀ����
����Ŀ��ij��ѧ��ȤС������Fe��CuSO4��Һ��Ӧʵ��ʱ����������Cu��ͬʱ�����ݲ�����Ϊ�˽�һ��̽�������ݲ���ԭ����������ʵ�飺
��ȡ8.5gFe�۷���һ������CuSO4�У���Ӧ��ɺ��˳����壬ϴ�ӡ�������������������Ϊ9.2g��
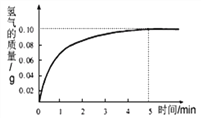
�ڽ���9.2g������һ������������ϡ�����ַ�Ӧ����������50.0g������ʵ�������ͼ��ʾ��Ӧʱ���뷴Ӧ����������������ϵͼ��
�����ṩ���й����ݽ������м���ͷ�����
(1)����ͼ���֪���������ϡ���ᷴӦ��Fe������Ϊ_______��
(2)�������CuSO4��Ӧ��Fe������Ϊ_______��
(3)�������CuSO4��Ӧ��Fe������������������ᷴӦ��Fe������֮��______(ѡ��������������С��������������)��ȡ��Fe������8.5g��
(4)������ʵ������ݷ����ó���Fe��CuSO4��Һ��Ӧ�������ݲ���ԭ��___________��
���𰸡� 2.8g 5.6g �� ��Ϊ��������������ͭ��Һ�е��������ʷ����˷�Ӧ(����ͭ��Һ�����ԣ������������䷴Ӧ
����������l���⣺����ϡ���ᷴӦ��Fe������Ϊx�����������Ϊw
Fe+2HCl�TFeCl2+H2��
56 73 2
x w 0.10g
![]()
��֮�ã�x=2.8g��w=3.65g��
����ϡ���ᷴӦ��Fe������Ϊ2.8g��
��2���⣺����CuSO4��Ӧ��Fe������Ϊy��
Fe+CuSO4�TFeSO4+Cu
56 64
y 9.2g-2.8g
![]()
��֮�ã�y=5.6g��
����CuSO4��Ӧ��Fe������Ϊ5.6g��
��3����CuSO4��Ӧ��Fe�������������ᷴӦ��Fe������֮��Ϊ��2.8g+5.6g=8.4g��8.5g��
��4����Fe��CuSO4��Һ��Ӧʵ��ʱ����������Cu��ͬʱ�����ݲ�����������CuSO4��Ӧ��Fe�������������ᷴӦ��Fe������֮�ͣ�8.5g��˵��������Fe��CuSO4��Һ�е��������ʷ����˷�Ӧ��������������