题目内容
【题目】构建知识网络图,是学会知识整理的重要方法。某同学整理的工业上炼铁炼钢和轧制钢材的主要流程如图:
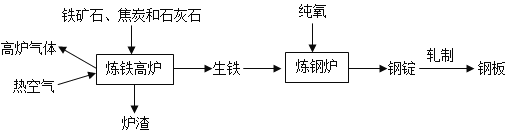
(1)高炉中铁矿石炼铁的化学方程式为___________________。
(2)炼铁的固体原料需经过粉碎,其目的是____________________。
(3)炉渣中含有硅酸钙(CaSiO3),其中硅元素的化合价是________。
(4)炼钢炉中,通入纯氧的目的是_____________________。将钢锭扎成钢板,体现了金属的________性。
(5)铁制品的生锈过程,实际上是铁与空气中的________________等发生化学反应的过程。写出用稀硫酸除锈发生反应的化学方程式____________________。
【答案】Fe2O3+3CO![]() 2Fe+3CO2 增大反应物接触面积,加快反应速率 +4 使生铁中碳充分反应,降低碳的含量 延展 O2和H2O 3H2SO4+Fe2O3=Fe2(SO4)3+3H2O
2Fe+3CO2 增大反应物接触面积,加快反应速率 +4 使生铁中碳充分反应,降低碳的含量 延展 O2和H2O 3H2SO4+Fe2O3=Fe2(SO4)3+3H2O
【解析】
本题主要考察工业炼铁的相关知识
(1)高炉中铁矿石炼铁反应原理:Fe2O3+3CO![]() 2Fe+3CO2
2Fe+3CO2
(2)增大反应物接触面积,使反应更加充分,加快反应速率
(3)硅元素的化合价为X,则(+2)+X+(-2)×3=0。X=+4
(4)炼钢炉中,通入纯氧的目的是使生铁中碳充分反应,降低碳的含量,钢锭扎成钢板,体现了金属有延展性
(5)铁在潮湿的空气中容易生锈,是铁和空气中的O2和H2O发生化学反应,稀硫酸除锈原理:3H2SO4+Fe2O3=Fe2(SO4)3+3H2O

练习册系列答案
相关题目