��Ŀ����
����Ŀ��ʵ���ҳ��õĸ������Ũ���ᡢ��ʯ��(CaO��NaOH�Ĺ�������)�ȣ������ڳ�ʪ�Ŀ������ױ��ʡ�ij��ѧ��ȤС���ʵ������һƿ���õļ�ʯ��չ��̽����
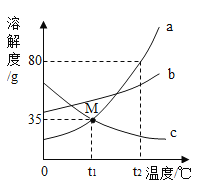
[��������]�ټ�ʯ�������տ����е�ˮ�����Ͷ�����̼ ���Ȼ�����Һ������,̼������Һ�ʼ��� ��̼���ƺ�������������ˮ�¶ȱ仯������ ��Ca(OH)2�ֽ��¶�Ϊ580�棬CaCO3�ֽ��¶�Ϊ825�棬Na2CO3�ķֽ��¶�Ϊ1744�档
[�������]��ʯ���Ƿ���ʣ���ɷֿ�������Щ��
[���в���]����û�б��ʣ���ʯ����ˮ�����ã��ɷ�ֻ��CaO��NaOH��
�������ʣ��ü�ʯ���п��ܺ���CaO��NaOH��Ca(OH)2��Na2CO3��CaCO3�е����ֻ��������ϡ�
[ʵ�����]
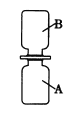
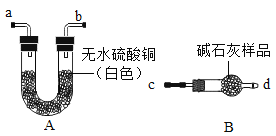
(1)��֤��ʯ���Ƿ���ʣ�ͼ�е�BΪ����װ�ã������ڹ��������������塣����A��Bװ�ü����ʯ���Ƿ���ʣ�ȡ��������ˮ����ͭ�ͼ�ʯ����Ʒ�ֱ�װ��A��B�У����Ӻ�A��Bװ�ã��� ____(����c������d��)����B�л���ͨ��ˮ�������۲�Aװ���е�����Ϊ____��֤����ʯ���ѱ��ʡ�
(2)��֤��ʯ���Ƿ���ȫ���ʣ�ȡ������ʯ����Ʒ�����Թ��У�������������ˮʹ�����ܽ⣬��Һ����ǣ����ִ����Թ���ڣ��¶������Ա仯��֤����ʯ������ȫ���ʡ�����Ʒ�ɷ������___�ֿ�����(������)��
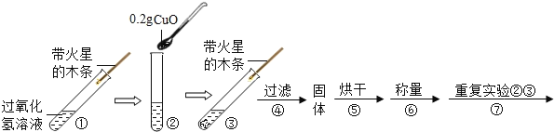
(3)Ϊ��һ��ȷ����ʯ����Ʒ�ijɷ֣���С�����ʵ�鲢��¼���£�
ʵ���� | ʵ����� | ʵ��Ŀ�ġ���������� | ʵ����� |
ʵ��һ | ��ȡ������Ʒ���Թ��У�������������ˮʹ�����ܽ⣻ �ڹ��ˣ��õ�����A����ҺB��������ҺB�м�������CaCl2��Һ�����ã� ��________�� | �����۵���ҪĿ���ǣ�___ �����ܵ�����____ | ��Ʒ��һ����Ca(OH)2 |
ʵ��� | ��ȡ������Ʒ50g��������600���ڣ��������������ٷ����仯����ȴ������� �ڽ�����ʣ����������850���ڷ������ȣ���ȴ������� | �������гƵù�������Ϊ45.5g�������ڹ��������ޱ仯�� | ��Ʒ��һ��û��_____(�ѧʽ) |
[̽������] ͨ������̽���������֪��ʯ����Ʒ�ijɷ���______(�ѧʽ)�������ε���������Ϊ_____��
[��˼������] ͨ��̽��������֪���ü�ʯ�ұ��ʵĻ�ѧ��Ӧ���̣����������εĻ�ѧ����ʽΪ______�������˼�ʯ��Ҫ�ܷⱣ���ԭ��
���𰸡�c ��ɫ��ˮ����ͭѸ�ٱ��� 3(���������ֻ��־���) ȡ���������ϲ���Һ�������Թ��У����Թ��еμӷ�̪��Һ(������Լ��ܼ����OH-����) ������Һ�е�̼�������(��̼����) ��Һ���(������Լ��ܼ���������������Ҷ�Ӧ������ȷ����) ![]()
![]() ��
�� ![]() 63%
63% ![]()
��������
(1) Aװ����װ����ˮ����ͭ��Bװ����װ�м�ʯ����Ʒ�����Ӻ�A��Bװ�ã������ʯ����Ʒ�Ƿ��и������ã���c����Bװ���л���ͨ��ˮ������d������Aװ�ã����۲�Aװ���е�����Ϊ��ɫ��ˮ����ͭѸ�ٱ�����˵����Ʒ��û�и������ã�֤����ʯ���ѱ��ʡ�
(2)ȡ������ʯ����Ʒ�����Թ��У�������������ˮʹ�����ܽ⣬��Һ����ǣ���������Ʒ����̼��ƶ��������ǣ�Ҳ��������Ʒ����̼���ƺ�������������ˮ��Ӧ����̼����������ִ����Թ���ڣ��¶������Ա仯��˵����Ʒ���������ƺ��������ƣ�֤����ʯ������ȫ���ʣ���Ʒ�ɷ���Ͽ�����![]() ��
��![]() ��
��![]() ����Ʒ�ɷ������3�ֿ����ԡ�
����Ʒ�ɷ������3�ֿ����ԡ�
��3��ȷ����Һû��̼���ƺ�������ɫ��̪�ж���Һ�Ƿ��������ƣ�����![]() �ֽ��¶�Ϊ580�棬
�ֽ��¶�Ϊ580�棬![]() �ֽ��¶�Ϊ825�棬
�ֽ��¶�Ϊ825�棬![]() �ķֽ��¶�Ϊ1744�棬������600�棬����������С������Ʒ��
�ķֽ��¶�Ϊ1744�棬������600�棬����������С������Ʒ��![]() ������������850���ڷ������ȣ������������䣬����Ʒһ������
������������850���ڷ������ȣ������������䣬����Ʒһ������![]() ������ˣ���Ʒһ����̼���ơ�
������ˣ���Ʒһ����̼���ơ�
ʵ���� | ʵ����� | ʵ��Ŀ�ġ���������� | ʵ����� |
ʵ��һ | ��ȡ������Ʒ���Թ��У�������������ˮʹ�����ܽ⣻ �ڹ��ˣ��õ�����A����ҺB��������ҺB�м�������CaCl2��Һ�����ã� ��ȡ���������ϲ���Һ�������Թ��У����Թ��еμӷ�̪��Һ�� | �����۵���ҪĿ���ǣ�������Һ�е�̼��������� �����ܵ�������Һ����� | ��Ʒ��һ����Ca(OH)2 |
ʵ��� | ��ȡ������Ʒ50g��������600���ڣ��������������ٷ����仯����ȴ������� �ڽ�����ʣ����������850���ڷ������ȣ���ȴ������� | �������гƵù�����Ϊ45.5g�������ڹ��������ޱ仯�� | ��Ʒ��һ��û�� |
[̽������] �ɣ�3������֪��ͨ������̽���������֪��ʯ����Ʒ�ijɷ���![]() ��
�� ![]() ���ȷֽ����������ƺ�ˮ��ʵ����й�����ٵ�����Ϊ����ˮ��ˮ����������������50g��Ʒ��
���ȷֽ����������ƺ�ˮ��ʵ����й�����ٵ�����Ϊ����ˮ��ˮ����������������50g��Ʒ��![]() ������Ϊx����
������Ϊx����
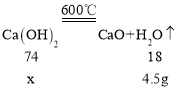
![]()
������Ʒ����![]() ����������Ϊ
����������Ϊ![]() ��
��
[��˼������] ͨ��̽������ü�ʯ�ұ��ʵĻ�ѧ��Ӧ������������![]() �Ļ�ѧ����ʽΪ
�Ļ�ѧ����ʽΪ![]() ���ʼ�ʯ��Ҫ�ܷⱣ�档
���ʼ�ʯ��Ҫ�ܷⱣ�档