��Ŀ����
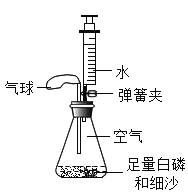
����Ŀ��ijʵ��С��������ͼ��ʾװ�ò����������������������ȡ�óɹ���
���������ϣ�����ȼ�յ�����¶���40�档
��������⣩�������Լռ����������Ķ��٣�
��ʵ��������ƿ�ڿ������Ϊ230mL��ע������ˮ�����Ϊ50mL����װ�����������ã��Ŵ���ˮ�����ձ���
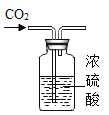
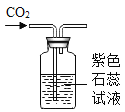
��ʵ��̽����װ��ҩƷ����ͼ��ʾ���Ӻ��������н����ɼС�
���������ۣ�
��1���ɽ���ƿ�а�����ȼ�ķ���_____��
��2������ƿ�ײ�������ˮ�У����ܿ챻��ȼ�������İ�������ƿ��δ��ȫ��ȼ�գ�˵��ƿ��ʣ������_____ȼ�գ�����֧����������֧��������д������ȼ�յķ��ű���ʽ��_____��
��3���ڰ���ȼ��ʱ��ȼ�պ���ȴ�����µĹ����У��ɹ۲쵽����ı仯���ȱ�_____���ٱ�_____�������ڴ�ʵ���е�������_____��
��4��������Ϩ����ƿ��ȴ�����º��ɼУ����ɹ۲쵽�������ǣ���ע�����е�ˮ�Զ�����������ڵ�ע�����е�ˮ��ʣԼ_____mLʱֹͣ������
���ó����ۣ�����Լռ�����������1/5��
����˼�����ۣ�
��5��ָ����װ����̲������ʵ����ȵ�һ���ŵ�_____��
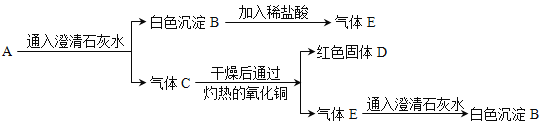
���𰸡�����ƿ�ײ�������ˮ�� ��֧�� P + O2 ![]() P2O5 �� С �����ã���ֹƿ������ 4 �ⶨ�������ȷ���Ի�������Ⱦ����
P2O5 �� С �����ã���ֹƿ������ 4 �ⶨ�������ȷ���Ի�������Ⱦ����
��������
��1������ȼ�յ�����¶���40�棬����ƿ�ײ�������ˮ�м��ɵ�ȼ���ף�
��2�������İ�������ƿ��δ��ȫ��ȼ�գ�˵��ƿ��ʣ�����岻֧��ȼ�գ�����ȼ�����ɵ������������ף���Ӧ�Ļ�ѧ���ű���ʽΪ��P + O2 ![]() P2O5��
P2O5��
��3������ȼ��ʱ�ų��������ȣ�ʹƿ��ѹǿ�������ڰ���ȼ������������������ȴ�����£�ƿ��ѹǿ��С���ɹ۲쵽����ı仯�ǣ��ȱ����С�������ڴ�ʵ���е������������ã���ֹƿ��������
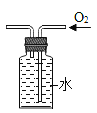
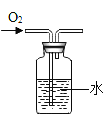
��4������ȼ����������ƿ�е�������ʹƿ����ѹ��С��ע�����е�ˮ�Զ����������ƿ������Լռ![]() ����ע�����е�ˮ������ƿ46mL��ƿ����ѹ������൱�����Ե�ע�����е�ˮ��ʣ��Լ4mLʱֹͣ������ͨ����ʵ����Եó����������Լռ�����������1/5��
����ע�����е�ˮ������ƿ46mL��ƿ����ѹ������൱�����Ե�ע�����е�ˮ��ʣ��Լ4mLʱֹͣ������ͨ����ʵ����Եó����������Լռ�����������1/5��
��5��װ��ʵ����̲�ʵ����ȣ�����װ�����ܷ�ģ����Բⶨ�������ȷ���Ի�������Ⱦ���١�

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ʵ���ҳ��õĸ������Ũ���ᡢ��ʯ��(CaO��NaOH�Ĺ�������)�ȣ������ڳ�ʪ�Ŀ������ױ��ʡ�ij��ѧ��ȤС���ʵ������һƿ���õļ�ʯ��չ��̽����
[��������]�ټ�ʯ�������տ����е�ˮ�����Ͷ�����̼ ���Ȼ�����Һ������,̼������Һ�ʼ��� ��̼���ƺ�������������ˮ�¶ȱ仯������ ��Ca(OH)2�ֽ��¶�Ϊ580�棬CaCO3�ֽ��¶�Ϊ825�棬Na2CO3�ķֽ��¶�Ϊ1744�档
[�������]��ʯ���Ƿ���ʣ���ɷֿ�������Щ��
[���в���]����û�б��ʣ���ʯ����ˮ�����ã��ɷ�ֻ��CaO��NaOH��
�������ʣ��ü�ʯ���п��ܺ���CaO��NaOH��Ca(OH)2��Na2CO3��CaCO3�е����ֻ��������ϡ�
[ʵ�����]
(1)��֤��ʯ���Ƿ���ʣ�ͼ�е�BΪ����װ�ã������ڹ��������������塣����A��Bװ�ü����ʯ���Ƿ���ʣ�ȡ��������ˮ����ͭ�ͼ�ʯ����Ʒ�ֱ�װ��A��B�У����Ӻ�A��Bװ�ã��� ____(����c������d��)����B�л���ͨ��ˮ�������۲�Aװ���е�����Ϊ____��֤����ʯ���ѱ��ʡ�
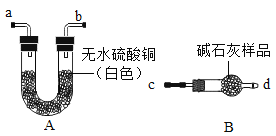
(2)��֤��ʯ���Ƿ���ȫ���ʣ�ȡ������ʯ����Ʒ�����Թ��У�������������ˮʹ�����ܽ⣬��Һ����ǣ����ִ����Թ���ڣ��¶������Ա仯��֤����ʯ������ȫ���ʡ�����Ʒ�ɷ������___�ֿ�����(������)��
(3)Ϊ��һ��ȷ����ʯ����Ʒ�ijɷ֣���С�����ʵ�鲢��¼���£�
ʵ���� | ʵ����� | ʵ��Ŀ�ġ���������� | ʵ����� |
ʵ��һ | ��ȡ������Ʒ���Թ��У�������������ˮʹ�����ܽ⣻ �ڹ��ˣ��õ�����A����ҺB��������ҺB�м�������CaCl2��Һ�����ã� ��________�� | �����۵���ҪĿ���ǣ�___ �����ܵ�����____ | ��Ʒ��һ����Ca(OH)2 |
ʵ��� | ��ȡ������Ʒ50g��������600���ڣ��������������ٷ����仯����ȴ������� �ڽ�����ʣ����������850���ڷ������ȣ���ȴ������� | �������гƵù�������Ϊ45.5g�������ڹ��������ޱ仯�� | ��Ʒ��һ��û��_____(�ѧʽ) |
[̽������] ͨ������̽���������֪��ʯ����Ʒ�ijɷ���______(�ѧʽ)�������ε���������Ϊ_____��
[��˼������] ͨ��̽��������֪���ü�ʯ�ұ��ʵĻ�ѧ��Ӧ���̣����������εĻ�ѧ����ʽΪ______�������˼�ʯ��Ҫ�ܷⱣ���ԭ��
����Ŀ����Ϣ�ӹ�����ָѧ���ڶ�ʱ������ѧ��֪ʶ,�ܶ���Ϣ������ȡ�ͼӹ�������
�����ϣ�
���ҹ������������ȼ�ƺ������ߵ�ú���γ�,�������ŷŵ�β��Ҳ����Ҫ����
����Һ�����ȳ�pH����ʾ,pHֵ��һ������0��14֮�����,��25����,��pH<7ʱ��Һ������,��ֵԽС����Խǿ:��pH>7ʱ,��Һ�ʼ���,��ֵԽ�����Խǿ;��pH=7ʱ,��Һ�����ԡ�
��ʵ�飩ij��ѧ����С��ȡ�ս����������ˮˮ��,��pH��(��pH������)ÿ��5���Ӳ�һ��pH,�����������ʾ,��ش���������
�ⶨʱ�� | 17:05 | 17:10 | 17:15 | 7:20 | 7:25 | 17:30 | 17:35 |
pH | 4.95 | 4.94 | 4.94 | 4.88 | 4.86 | 4.85 | 4.85 |
(1)�ڲⶨ�ڼ�,����ˮΪ____________(��������������������������),������________��������ǿ����������������������
(2)����17:40ʱ�̲ⶨ,pHӦΪ____________(����ĸ)
A 4.87 B 4.86 C 4.84 D 4.00
��Ӧ�ã�
(3)�����һ����������Ĵ�ʩ________________________
(4)���ն������������������,��ѧ����ʽΪ2SO2+O2+2H2O=2H2SO4,�μӷ�Ӧ��SO2�����ɵ�H2SO4����������____________������3.2t��S��ȫȼ��,ȫ��ת��,�ɵõ�H2SO4������Ϊ____________t��